10-K: Annual report pursuant to Section 13 and 15(d)
Published on March 14, 2008
QuickLinks -- Click here to rapidly navigate through this document
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
ý ANNUAL REPORT PURSUANT TO SECTION 13 OR 15 (d) OF
THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2007
Or
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15 (d) OF THE
SECURITIES AND EXCHANGE ACT OF 1934 (NO FEE REQUIRED)
Commission File Number 0-26866
Sonus Pharmaceuticals, Inc.
(Exact name of the registrant as specified in its charter)
| Delaware | 95-4343413 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
1522 217th Place SE, Suite 100, Bothell, Washington 98021
(Address of principal executive offices)
(425) 487-9500
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Name of Exchange on Which Registered |
|
|---|---|---|
| Common Stock, par value $0.001 per share Series A Junior Participating Preferred Stock, par value $0.001 per share |
The NASDAQ Stock Market, LLC The NASDAQ Stock Market, LLC |
Securities
registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 of 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such report), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act.). Yes o No ý
As of June 30, 2007, the aggregate market value of the registrant's Common Stock held by non-affiliates of the registrant was $173,157,479. As of March 3, 2008, 37,047,335 shares of the registrant's Common Stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive Proxy Statement to be filed in connection with the solicitation of proxies for its 2008 Annual Meeting of Stockholders are incorporated by reference in Items 10, 11, 12, 13 and 14 of Part III hereof.
Sonus Pharmaceuticals, Inc.
Table of Contents
| PART I | |||||
| ITEM 1. | BUSINESS | 3 | |||
| Overview | 3 | ||||
| Product Candidates | 3 | ||||
| Research and Development Pipeline | 4 | ||||
| Market Overview | 4 | ||||
| Collaboration and License Agreement with Bayer Schering Pharma AG | 4 | ||||
| Research and Development | 4 | ||||
| Government RegulationsDrug Approval Process | 5 | ||||
| Competition | 6 | ||||
| Patents and Proprietary Rights | 7 | ||||
| Product Liability | 8 | ||||
| Employees | 8 | ||||
| Company Information | 8 | ||||
| ITEM 1A. | RISK FACTORS | 9 | |||
| ITEM 1B. | UNRESOLVED STAFF COMMENTS | 17 | |||
| ITEM 2. | PROPERTIES | 18 | |||
| ITEM 3. | LEGAL PROCEEDINGS | 18 | |||
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS | 18 | |||
|
PART II |
|||||
| ITEM 5. | MARKET FOR THE REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 18 | |||
| ITEM 6. | SELECTED FINANCIAL DATA | 20 | |||
| ITEM 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 20 | |||
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 28 | |||
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 30 | |||
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 53 | |||
| ITEM 9A. | CONTROLS AND PROCEDURES | 53 | |||
| ITEM 9B. | OTHER INFORMATION | 56 | |||
|
PART III |
|||||
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 56 | |||
| ITEM 11. | EXECUTIVE COMPENSATION | 56 | |||
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 56 | |||
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 57 | |||
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES | 57 | |||
|
PART IV |
|||||
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | 57 | |||
ii
References in this Form 10-K to "Sonus Pharmaceuticals", "Sonus", the "Company", "we", "us" or "our" refer to Sonus Pharmaceuticals, Inc. The information in this Form 10-K contains certain forward-looking statements, including statements related to clinical trials, regulatory approvals, markets for the Company's products, new product development, capital requirements and trends in its business that involve risks and uncertainties. The Company's actual results may differ materially from the results discussed in the forward-looking statements. Factors that might cause such a difference include those discussed in "Business", "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" as well as those discussed elsewhere in this Form 10-K.
Overview
Sonus Pharmaceuticals is developing novel small molecule drugs for the treatment of patients with cancer. Our objective is to identify opportunities where there is the possibility for major improvements in patient treatments and where we believe we can mitigate development risk. We currently have one drug in clinical development and two earlier stage programs. Our plan is to continue to develop our internal pipeline of clinical compound opportunities and to evaluate possible strategic alternatives, including in-licensing, out-licensing and merger and acquisition opportunities, as a means of achieving our business strategies and enhancing stockholder value. In the fourth quarter of 2007 we engaged Ferghana Partners Inc., an international provider of independent financial advisory services to firms in the biotechnology, pharmaceuticals, diagnostics and specialty chemicals industries, to assist us with these strategic alternatives.
Product Candidates
SN2310
SN2310 Injectable Emulsion ("SN2310") is a novel camptothecin derivative. Camptothecins are an important class of anti-cancer drugs introduced in recent years; however, the marketed camptothecin analogs, irinotecan (Camptosar®) and topotecan (Hycamtin®), have demonstrated limitations that may reduce their clinical utility. Irinotecan and topotecan are used in the treatment of colorectal, lung, and ovarian cancers. SN2310 is a prodrug of SN-38. SN-38 is also the active moiety of irinotecan. Our objective with SN2310 is to provide a product that has enhanced anti-tumor activity and improved tolerability compared with the approved camptothecin-based products, and is ready-to-use. An Investigational New Drug Application ("IND") was submitted to the U.S. Food and Drug Administration ("FDA") for SN2310 in June 2006 and Phase 1 clinical testing was initiated in September 2006. We expect to close enrollment in this study and initiate a Phase 2 clinical trial in 2008. As this product candidate is early in clinical development, we cannot give any assurance that this compound will be clinically successful.
TOCOSOL® Paclitaxel
TOCOSOL Paclitaxel is a novel formulation of paclitaxel manufactured in a ready-to-use, injectable vitamin E-based emulsion formulation. On September 24, 2007 we announced that TOCOSOL Paclitaxel failed to meet the primary endpoint in Phase 3 clinical testing. We have discontinued development of TOCOSOL Paclitaxel due to results of the Phase 3 study, and the time and cost that would be required to conduct the necessary clinical studies to continue development, which at a minimum, would include another Phase 3 pivotal trial. These closure activities were substantially complete by December 31, 2007.
3
Research and Development Pipeline
We continue to invest in the research and development of new oncology related product candidates. We have identified two areas of opportunity where we believe there is the possibility for major improvements in patient treatments and where we believe we can mitigate development risk: (1) prodrugs of existing small molecules, where the novel prodrug is designed to provide greater patient convenience and improved patient outcome; and (2) novel small molecules, where an opportunity exists, using known moieties, to address clinical shortcomings of existing approved compounds. From these programs, Sonus has identified inhibitors of DNA methyl transferase and expects to select a lead compound for further development in the second half of 2008. DNA methyl transferase inhibitors, such as the approved drugs azacytidine and decitabine, act by normalizing the expression of repressed genes, leading to apoptosis (cell death) of malignant cells.
Market Overview
Cancer is characterized by rapid, uncontrolled cell division resulting in the growth of an abnormal mass of cells generally referred to as a malignant tumor. Cancerous tumors can arise in almost any tissue or organ, and cancer cells, if not eradicated, can spread, or metastasize, throughout the body. As these tumors grow, they cause damage to the surrounding tissue and organs and commonly result in death if left untreated. Cancer is believed to occur as a result of a number of hereditary and environmental factors. According to the American Cancer Society, cancer is the second leading cause of death in the United States and accounts for approximately one in every four deaths. Approximately 565,000 Americans are expected to die of cancer in 2008. The National Institutes of Health estimated the direct medical cost of cancer to be $89 billion in 2007.
Product candidates in our pipeline are in the early stages of development, and it is difficult to evaluate the potential markets for these product candidates as the areas of potential application are diverse and specific applications are yet to be determined.
Collaboration and License Agreement with Bayer Schering Pharma AG
On October 17, 2005, we entered into a License and Collaboration Agreement (the "Bayer Agreement") with Bayer Schering Pharma AG (formerly Schering AG), a German corporation, pursuant to which, among other things, we granted Bayer Schering an exclusive, worldwide license to TOCOSOL Paclitaxel. On October 3, 2007, we received notification from Bayer Schering of its decision to terminate the Bayer Agreement in accordance with its terms because the Phase 3 pivotal trial did not meet its primary endpoint and the results of the trial did not support, in Bayer Schering's judgment, a submission for a New Drug Application with the FDA. In accordance with the terms of the Bayer Agreement, all rights to TOCOSOL Paclitaxel have reverted to us. We do not expect recognition of any revenue or expense related to the Bayer Agreement beyond December 31, 2007.
Research and Development
We currently conduct research and development activities at our facilities in Bothell, Washington. We also engage in certain research, preclinical studies and clinical development efforts at third party laboratories and other institutions. Our primary research and development efforts are currently directed at the development of SN2310 and two other areas of research where we can use our expertise and technology to improve the safety or efficacy of oncology drugs.
Our research and development activities for the last three years can be divided into research, preclinical and clinical development programs primarily associated with TOCOSOL Paclitaxel as well as research, preclinical and clinical activities related to our other early stage product candidates.
4
The approximate costs associated with these programs for the last three fiscal years were as follows (in millions):
|
|
2007 |
2006 |
2005 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| TOCOSOL Paclitaxel | $ | 17.9 | $ | 32.4 | $ | 21.2 | |||
| Other clinical, preclinical and research programs | $ | 9.2 | $ | 8.4 | $ | 3.0 | |||
| Total research & development | $ | 27.1 | $ | 40.8 | $ | 24.2 | |||
We separately tracked all billable costs associated with TOCOSOL Paclitaxel as it was our lead product candidate and had been partnered with Bayer Schering. Costs attributed to other clinical, preclinical and research projects largely represent our pipeline generating activities. See Item 7. "Management's Discussion and Analysis of Financial Condition and Results of Operations" for further discussion of research and development spending trends.
Government RegulationsDrug Approval Process
Regulation by governmental authorities in the U.S. and other countries is a significant factor in our ongoing research and development activities and in the production and marketing of our products. In order to undertake clinical tests, to produce and market products for human use, mandatory procedures and safety standards, established by the FDA in the U.S. and by comparable agencies in other countries, must be followed.
The standard process before a pharmaceutical agent may be marketed includes the following steps:
-
- Preclinical
studies including laboratory evaluation and animal studies to test for initial safety and efficacy;
-
- Submission
to national health authorities of an IND, or Clinical Trials Application ("CTA") or equivalent dossier, which must be accepted by each national health authority
before human clinical trials may commence in that country;
-
- Adequate
and well-controlled clinical trials to establish the safety and efficacy of the drug in its intended population and use(s);
-
- Submission
to appropriate national and/or regional regulatory health authorities of a New Drug Application (NDA), or equivalent marketing authorization application, which
application is not automatically accepted for review; and
-
- approval by appropriate regulatory health authorities of the marketing authorization application prior to any commercial sale or shipment of the drug in each country or jurisdiction.
As part of the regulatory health authority approval for each product, the drug-manufacturing establishment is subject to inspection by the FDA and must comply with current Good Manufacturing Practices ("cGMP") requirements applicable to the production of pharmaceutical drug products. The facilities, procedures, and operations of manufacturers must be determined to be adequate by the FDA before product approval.
Preclinical studies include laboratory evaluation of the active drug substance and its formulation in animal studies to assess the potential safety and efficacy of the drug and its formulation. Prior to initiating the first clinical testing of a new drug product candidate, the results of the preclinical studies are submitted to regulatory health authorities as part of an IND or CTA, and must be accepted before the proposed clinical trial(s) can begin.
Clinical trials for cancer therapeutics involve the administration of the investigational drug product to patients with a defined disease state, under the supervision of a qualified principal investigator.
5
Clinical trials are conducted in accordance with protocols that detail the parameters to be used to monitor safety and efficacy. Each protocol is submitted to regulatory health authorities as part of the IND/CTA, in each country where clinical trials are to be conducted. Each clinical study is approved and monitored by independent Institutional Review Boards or Ethics Committees who consider ethical factors, informed consent documents, the safety of human subjects and the possible liability of the institutions conducting a clinical study. The Institutional Review Board or Ethics Committee may require changes in the clinical trials protocol, which may delay initiation or completion of the study.
Clinical trials typically are conducted in three sequential phases, although the phases may overlap. In Phase 1, the initial introduction of the drug to humans, the drug is tested for safety and clinical pharmacology. Phase 2 trials involve more detailed evaluation of the safety and efficacy of the drug in patients with a defined disease. Phase 3 trials consist of large scale evaluations of safety and efficacy of the investigational product compared to accepted standard therapy in a defined disease.
The process of completing clinical testing and obtaining regulatory health authority approval for a new product takes a number of years and requires the expenditure of substantial resources. Regulatory health authorities may conclude that the data submitted in a marketing authorization application are not adequate to support an approval and may require further clinical and preclinical testing, re-submission of the application, and further review. Even after initial approval has been obtained, further studies may be required to provide additional data about the approved indication, and further studies will be required to gain approval for the use of a product for clinical indications other than those for which the product was approved initially. Also, health authorities require post-marketing surveillance programs to monitor the drug product's side effects.
Marketing of pharmaceutical products outside of the U.S. is subject to regulatory requirements that vary from country to country. In the European Union, the general trend has been towards coordination of common standards for clinical testing of new drug products. Centralized approval in the European Union is coordinated through the European Medicines Agency, or EMEA.
The level of regulation outside the U.S. and European Union varies widely. The time required to obtain regulatory approval from regulatory agencies in each country may be longer or shorter than that required for FDA or EMEA approval. In addition, in certain markets, reimbursement is subject to governmentally mandated prices.
Many of the chemicals and compounds used in our research and development efforts are classified as hazardous materials under applicable federal, state and local environmental laws and regulations. We are subject to regulations under state and federal law regarding occupational safety, laboratory practices, handling and disposing of chemicals, environmental protection and hazardous substance control.
Competition
The healthcare industry in general is characterized by extensive research efforts, rapid technological change and intense competition. We believe that other pharmaceutical companies will compete with us in areas of research and development, acquisition of products and technology licenses, and the manufacturing and marketing of products that could potentially compete with ours. We expect that competition will be based on safety, efficacy, ease of administration, breadth of approved indications, price, reimbursement and physician and patient acceptance.
The two approved camptothecins are irinotecan and topotecan with combined 2006 sales in excess of $1.1 billion. These products are approved for the treatment of metastatic colorectal, small cell lung and ovarian cancer. Irinotecan came off patent in February 2008.
6
We believe that our ability to successfully compete in the biotechnology and pharmaceutical industries will be based on our ability to do the following:
-
- Develop
proprietary products;
-
- Attract
and retain key scientific personnel;
-
- Obtain
patent or other protection for products;
-
- Obtain
required regulatory approvals; and
-
- Manufacture, market and or license our products alone or with collaborative partners.
Many of our competitors and potential competitors have substantially greater financial, technical and human resources than we do and have substantially greater experience in developing products, obtaining regulatory approvals and marketing and manufacturing products. Companies that complete clinical trials, obtain required regulatory approvals and commence commercial sales of their products before their competitors may achieve a significant competitive advantage if their products work through a similar mechanism as our products. In addition, other technologies or products may be developed that have an entirely different approach that would render our technology and products noncompetitive or obsolete.
Patents and Proprietary Rights
We consider the protection of our technology to be important to our business. In addition to seeking U.S. patent protection for our inventions, we are also seeking patent protection in other selected countries in order to broadly protect our proprietary rights. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position.
Our success will depend, in part, on our ability to obtain and defend patents and protect trade secrets. As of December 31, 2007, sixteen United States patents have been issued to Sonus. A composition of matter patent covering SN2310 issued in the United States in 2007, and national stage applications have been filed in key countries. Nine patents pertaining to our proprietary TOCOSOL technology have issued in the U.S., and TOCOSOL-related patents have also issued in Europe, Canada, Taiwan, Mexico, Korea, and India. Additional patent applications covering our research programs are pending in the U.S. and other countries.
The patent position of medical and pharmaceutical companies is highly uncertain and involves complex legal and factual questions. There can be no assurance that any claims which are included in pending or future patent applications will be issued, that any issued patents will provide us with competitive advantage or will not be challenged by third parties, or that existing or future patents of third parties will not have an adverse effect on our ability to commercialize our products. Furthermore, there can be no assurance that other companies will not independently develop similar products, duplicate any of our products or design around patents that may be issued to us. Litigation or administrative proceedings may be necessary to enforce any patents issued to us or to determine the scope and validity of others' proprietary rights.
Our commercial success will depend in part on not infringing patents issued to competitors. There can be no assurance that patents belonging to competitors or others will not require us to alter our products or processes, pay licensing fees or cease development of our current or future products. Further, there can be no assurance that we will be able to license other technology that we may require at a reasonable cost or at all. Failure by us to obtain a license to any technology that we may require to commercialize our products could have a material adverse effect on our business, financial condition and results of operations.
7
We have obtained registration for our trademarks TOCOSOL® and Sonus Pharmaceuticals® in the United States and in a number of foreign countries. There can be no assurance that the registered or unregistered trademarks or trade names of our company will not infringe upon third party rights or will be acceptable to regulatory agencies.
We also rely on unpatented trade secrets, proprietary know-how and continuing technological innovation, which we seek to protect, in part, by confidentiality agreements with our corporate partners, collaborators, employees and consultants in our drug development research. There can be no assurance that these agreements will not be breached, that we will have adequate remedies for any breach, or that our trade secrets or know-how will not otherwise become known or be independently discovered by competitors. Further, there can be no assurance that we will be able to protect our trade secrets or that others will not independently develop substantially equivalent proprietary information and techniques.
Product Liability
The testing, marketing and sale of pharmaceutical products entails a risk of product liability claims by consumers and others. We currently maintain product liability insurance for our clinical trials with limits of $10 million per claim and in the aggregate, which we believe to be adequate for current non-commercial applications of our products. In the event of a successful suit against us, the lack or insufficiency of insurance coverage could have a material adverse effect on our business and financial condition. Although we have never been subject to a product liability claim, there can be no assurance that the coverage limits of our insurance policies will be adequate or that one or more successful claims brought against us would not have a material adverse effect upon our business, financial condition and results of operations. If any of our products under development gain marketing approval from the FDA or other regulatory health authorities, there can be no assurance that adequate product liability insurance will be available, or if available, that it will be available at a reasonable cost. Any adverse outcome resulting from a product liability claim could have a material adverse effect on our business, financial condition and results of operations.
Employees
As of March 1, 2008, we had 48 employees, including five who work part-time. Of these, 30 were engaged in research and development, regulatory, clinical and manufacturing activities, and 18 in business operations and administration. All of our employees are covered by confidentiality agreements. We consider our relations with our employees to be good, and none of our employees is a party to a collective bargaining agreement.
On November 1, 2007, the Company implemented a reduction of workforce ("Reduction of Workforce") pursuant to which the Company's workforce was reduced by 16 positions, or approximately 25%. The effective date of the Reduction of Workforce was November 30, 2007. The Company undertook the Reduction of Workforce in light of the outcome of its Phase 3 Pivotal Trial for TOCOSOL Paclitaxel. These steps were taken in order to conserve cash and preserve the critical capabilities necessary to pursue the highest priority development programs.
In connection with the Reduction of Workforce, the Company incurred expenses associated with one-time termination benefits of approximately $1.2 million, including approximately $1.1 million of severance benefits and $100,000 attributable to the continuation of medical insurance benefits. These expenses were recorded in the fourth quarter of 2007.
Company Information
Sonus Pharmaceuticals was incorporated in California in October 1991 and subsequently reorganized as a Delaware corporation in September 1995. The Company's principal executive offices are located at 1522 217th Place SE, Suite 100, Bothell, Washington 98021, and its telephone number is (425) 487-9500.
8
The Company makes its annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to these reports available on its website, at http://www.sonuspharma.com, free of charge as soon as practicable after filing with the SEC. All such reports are also available free of charge via EDGAR through the SEC website at www.sec.gov. In addition, the public may read and copy materials filed by the Company with the SEC at the SEC's public reference room located at 450 Fifth St., N.W., Washington, D.C., 20549. Information regarding operation of the SEC's public reference room can be obtained by calling the SEC at 1-800-SEC-0330.
You should consider the risks below carefully in addition to other information contained in this report before engaging in any transaction involving shares of our common stock. If any of these risks occur, they could seriously harm our business, financial condition or results of operations. In such case, the trading price of our common stock could decline, and you may lose all or part of your investment. We undertake no obligation to publicly release the results of any revisions to any forward-looking statements to reflect anticipated or unanticipated events or circumstances occurring after the date of such statements.
We will need additional capital in the future to support the continued development of our product candidates and to fund continuing operations.
Although, we expect that our cash requirements will decrease in future periods due to the discontinuation of development of TOCOSOL Paclitaxel, we will need additional capital in 2009 to support the continued development of SN2310, other product candidates and to fund continuing operations. We believe that existing cash, cash equivalents and marketable securities will be sufficient to fund current operations into the third quarter of 2009. Our future capital requirements depend on many factors including:
-
- our
ability to obtain, and the timing of payments under, debt or equity financings;
-
- timing
and costs of preclinical development, clinical trials and regulatory approvals;
-
- timing
and cost of drug discovery and research and development;
-
- entering
into new collaborative or product license agreements for products in our pipeline; and
-
- costs related to obtaining, defending and enforcing patents.
Any future debt or equity financing, if available, may result in substantial dilution to existing stockholders, and debt financing, if available, may include restrictive covenants.
We may merge with or acquire other companies or drug candidates, and our failure to receive the anticipated benefits in these transactions could harm our business.
We are actively seeking strategic opportunities, which may include a merger or acquisition, among other things. The success of any merger or acquisition depends, in part, on our ability to realize the anticipated synergies, cost savings and growth opportunities from integrating the business of the merged or acquired company with our business. The integration of two independent companies is a complex, costly and time-consuming process. The difficulties of combining the operations of two companies include, among others:
-
- consolidating
research and development operations;
-
- retaining
key employees;
-
- consolidating
corporate and administrative infrastructures;
-
- preserving the research and development and other important relationships of the companies;
9
-
- integrating
and managing the technology of two companies;
-
- using
the merged or acquired company's liquid capital and other assets efficiently to develop the business of the combined company;
-
- diverting
management's attention from ongoing business concerns; and
-
- coordinating geographically separate organizations.
There can be no assurance that we will find any attractive strategic opportunities, or that if we find them, that we will be able to consummate a transaction on favorable terms, or at all. If we do enter into a transaction, there can be no assurance that we will receive all of the anticipated benefits of any transaction, or that any of the risks described above will not occur. Our failure to receive anticipated benefits of and our exposure to inherent risks in, any such transaction could significantly harm our business, financial condition and operating results.
Failure to satisfy NASDAQ Global Market listing requirements may result in our common stock being delisted from The NASDAQ Global Market.
Our common stock is currently listed on The NASDAQ Global Market under the symbol "SNUS." For continued inclusion on The NASDAQ Global Market, we must maintain, among other requirements, stockholders' equity of at least $10.0 million, a minimum bid price of $1.00 per share and a market value of our public float of at least $5.0 million; or market capitalization of at least $50 million, a minimum bid price of $1.00 per share and a market value of our public float of at least $15.0 million. The closing price of our common stock, as reported on the NASDAQ Global Market as of March 3, 2008 was $0.45 per share. On November 5, 2007, we received notice from NASDAQ that we do not comply with NASDAQ's continued listing standards because the closing bid price of our common stock has been below the required minimum bid price of $1.00 for 30 consecutive business days. We have until May 5, 2008 to regain compliance with the minimum bid price requirement. If we do not regain compliance by May 5, 2008, our common stock will be delisted if we do not appeal NASDAQ's determination to delist our common stock. Alternatively, we may apply for listing on The NASDAQ Capital Market if we meet the initial listing standards for that market, in which case we would have an additional 180 days to regain compliance. In addition, as of December 31, 2007, we had stockholders' equity of approximately $31.9 million. In the event that we fail to satisfy any of the listing standards on a continuous basis, our common stock may be removed from listing on The NASDAQ Global Market. If our common stock were delisted from The NASDAQ Global Market and we are unable to transfer to The NASDAQ Capital Market, trading of our common stock, if any, may be conducted in the over-the-counter market in the so-called "pink sheets" or, if available, the OTC Bulletin Board. Consequently, broker-dealers may be less willing or able to sell and/or make a market in our common stock. Additionally, an investor would find it more difficult to dispose of, or to obtain accurate quotations for the price of, our common stock. As a result of a delisting from The NASDAQ Global Market, it may become more difficult for us to raise funds through the sale of our securities.
The success of our potential products in research and preclinical studies does not guarantee that these results will be replicated in humans.
Several of our drug development programs are currently in the research stage or in preclinical development. Our only product in clinical trials, SN2310, began Phase 1 clinical testing in September 2006 and is still in the early stages of clinical testing. Although our clinical development-stage drug candidate has shown favorable results in preclinical studies, these results may not be replicated in our clinical trials. Before we make any products from our research and development programs commercially available, we will need to conduct further research and development, including laboratory testing, animal studies, clinical studies, and obtain product approval from the appropriate regulatory authorities. These programs may not move beyond their current stages of development. Even if our research does advance, we will need to engage in certain additional preclinical development efforts to
10
determine whether a product is sufficiently safe and effective to enter clinical trials. Consequently, there is no assurance that the results in our research and preclinical studies are predictive of the results that we may see in our clinical trials, that they are predictive of whether any resulting products will be safe and effective in humans, or that the resulting products will be approved by regulatory authorities.
Our success is dependent on the proper management of our current and future business operations, and the expenses associated with them given our limited resources.
Our business strategy requires us to manage our operations to provide for the continued development and potential commercialization of our drug candidates. If we are unable to effectively manage our current operations given our limited resources, we may not be able to implement our business strategy and our financial condition and results of operations may be adversely affected. If we are unable to effectively manage our expenses, we may find it necessary to reduce our expenses through a reduction in our workforce and/or cancellation of research & development programs, which could adversely affect our operations.
We may never realize revenue from product commercialization.
Most of our attention and resources at this time are directed to the development of SN2310, a novel camptothecin derivative, as well as earlier stage oncology product candidates. Significant expenditures in additional research and development, clinical testing, regulatory, manufacturing, and sales and marketing activities will be necessary in order for us to gain marketing approval for our product candidates and subsequently commercialize them. There can be no assurance that product candidates under development or any future products will be safe and efficacious. If the product candidates under development are ultimately ineffective in treating cancer, do not receive the necessary regulatory approvals or do not obtain commercial acceptance, we will incur additional losses, our accumulated deficit will increase and our business will be materially adversely affected.
Even if we are successful in developing our products, there is no assurance that such products will receive regulatory approval or that a commercially viable market will develop.
We may not achieve our projected development goals in the time frames we announce and anticipate.
We set goals for and make public statements regarding the timing of certain accomplishments, such as the commencement and completion of clinical trials, anticipated regulatory approval dates and time of product launch, which we sometimes refer to as milestones. These milestones may not be achieved, and the actual timing of these events can vary dramatically due to a number of factors such as delays or failures in our clinical trials, disagreements with future collaborative partners, the uncertainties inherent in the regulatory approval process and manufacturing scale-up and delays in achieving manufacturing or marketing arrangements sufficient to commercialize our products. There can be no assurance that our clinical trials will be completed, that we will make regulatory submissions or receive regulatory approvals as planned or that we will be able to launch any of our products in anticipated timeframes. If we fail to achieve one or more of these milestones as planned, our business will be materially adversely affected, and the price of our shares will decline.
We have not yet commercialized any of our drug candidates; our ability to commercialize products is unproven.
We have not yet commercialized any of our product candidates. Our commercialization of products is subject to several risks, including but not limited to:
-
- the
possibility that a product is toxic, ineffective or unreliable;
-
- failure
to obtain regulatory approval for the product;
-
- difficulties in manufacturing the product on a large scale;
11
-
- difficulties
in planning, coordinating and executing the commercial launch of the product;
-
- difficulties
in marketing, distribution or sale of the product;
-
- the
possibility of a failure to comply with laws and regulations related to the marketing, sale and reimbursement of the product;
-
- competition
from superior products; and
-
- third-party patents that preclude us from marketing a product.
Even if a product candidate is approved for commercial sale, significant strategic planning and resources will be necessary to effectively coordinate commercial launch of the product in the approved indication or indications, and to effectively market, distribute and sell the product for use in the approved indication or indications. We currently have limited marketing and no distribution capability.
We have a history of operating losses which we expect will continue and we may never become profitable.
We have experienced significant accumulated losses since our inception, and expect to incur net losses for the foreseeable future. These losses have resulted primarily from expenses associated with our research and development activities, including nonclinical and clinical trials, and general and administrative expenses. As of December 31, 2007, our accumulated deficit totaled $124.8 million. We anticipate that our operating losses will continue as we further invest in research and development for our products. Our results of operations have varied and will continue to vary significantly and depend on, among other factors:
-
- our
ability to obtain and timing of payments under debt or equity financings;
-
- outcome
related to strategic activities currently being evaluated;
-
- timing
and costs of preclinical development, clinical trials and regulatory approvals;
-
- drug
discovery and research and development;
-
- entering
into new collaborative or product license agreements for products in our pipeline; and
-
- costs related to obtaining, defending and enforcing patents.
Governmental regulatory requirements are lengthy and expensive and failure to obtain necessary approvals will prevent us from commercializing a product.
We are subject to uncertain governmental regulatory requirements and a lengthy approval process for our products prior to any commercial sales of our products. The development and commercial use of our products are regulated by the FDA, the EMEA, and comparable regulatory agencies in other countries. The regulatory approval process for new products is lengthy and expensive. Before we can submit an application to the FDA and comparable international agencies, the product candidate must undergo extensive testing, including animal studies and human clinical trials that can take many years and require substantial expenditures. Data obtained from such testing may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. In addition, changes in regulatory policy for product approval may cause additional costs in our efforts to secure necessary approvals.
Our product candidates are subject to significant uncertainty because they are in early stages of development and are subject to regulatory approval. The results of preclinical and clinical testing of our products are uncertain and regulatory approval of our products may take longer or be more expensive than anticipated, which could have a material adverse effect on our business, financial condition and results of operations. We cannot predict if or when any of our products under development will be commercialized.
12
If we lose our key personnel or are unable to attract and retain qualified scientific and management personnel, we may be unable to become profitable.
The loss of any key employees or the inability to recruit and retain qualified scientific personnel to perform research and development and qualified management personnel could have a material adverse effect on our business, financial condition and results of operations. There can be no assurance that we will be able to attract and retain such personnel on acceptable terms, if at all, given the competition for experienced scientists and other personnel among numerous medical and pharmaceutical companies, universities and research institutions. We are highly dependent on our key executives, including Michael A. Martino, President & Chief Executive Officer and Alan Fuhrman, Senior Vice President & Chief Financial Officer, both of whom have Change in Control Agreements with the Company.
Future U.S. or international legislative or administrative actions also could prevent or delay regulatory approval of our products.
Even if regulatory approvals are obtained, they may include significant limitations on the indicated uses for which a product may be marketed. A marketed product also is subject to continual FDA, EMEA and other regulatory agency review and regulation. Later discovery of previously unknown problems or failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market, as well as possible civil or criminal sanctions. In addition, if marketing approval is obtained, the FDA, EMEA or other regulatory agency may require post-marketing testing and surveillance programs to monitor the product's efficacy and side effects. Results of these post-marketing programs may prevent or limit the further marketing of a product.
The development of oncology related pharmaceutical products is extremely competitive, and if we fail to compete effectively, it would negatively impact our business.
Competition in the development of pharmaceutical products is intense and expected to increase. We also believe that other medical and pharmaceutical companies will compete with us in the areas of research and development, acquisition of products and technology licenses, and the manufacturing and marketing of our products. Success of products in these fields will be based primarily on:
-
- efficacy;
-
- safety;
-
- price;
-
- breadth
of approved indications; and
-
- physician, healthcare payor and patient acceptance.
Many of our competitors and potential competitors, including large pharmaceutical, chemical and biotechnology concerns and universities and other research institutions, have substantially greater financial, technical and human resources than we do and have substantially greater experience in developing products, obtaining regulatory approvals and marketing and manufacturing pharmaceutical products. Accordingly, these competitors may succeed in obtaining FDA approval for their products more rapidly than we do. In addition, other technologies or products may be developed that have an entirely different approach that would render our technology and products noncompetitive or obsolete. If we fail to compete effectively, it would have a material adverse effect on our business, financial condition and results of operations.
13
We rely on third party suppliers and manufacturers to produce products that we develop and failure to retain such suppliers and manufacturers would adversely impact our ability to commercialize our products.
We currently rely on third parties to supply the chemical ingredients necessary for our drug product candidates. The chemical ingredients for our products are manufactured by a limited number of vendors. The inability of these vendors to supply medical-grade materials to us could delay the manufacturing of, or cause us to cease the manufacturing of our products. We also rely on third parties to manufacture our products for research and development and clinical trials. Suppliers and manufacturers of our products must operate under cGMP regulations, as required by the FDA, and there are a limited number of contract manufacturers that operate under cGMP regulations. cGMP are enumerated in FDA regulations and guidance documents. The facilities, procedures, and operations of our contract manufacturers must be determined to be adequate by the FDA before approval of product manufacturing for commercial use. Manufacturing facilities are subject to inspections by the FDA for compliance with cGMP, licensing specifications, and other FDA regulations. Failure to comply with FDA and other governmental regulations can result in fines, unanticipated compliance expenditures, recall or seizure of products, total or partial suspension of production and/or distribution, suspension of the FDA's review of NDAs, injunctions and criminal prosecution. Any of these actions could have a material adverse effect on us. Our reliance on independent manufacturers involves a number of other risks, including the absence of adequate capacity, the unavailability of, or interruptions in, access to necessary manufacturing processes and reduced control over delivery schedules. If our manufacturers are unable or unwilling to continue manufacturing our products in required volumes or have problems with commercial scale-up, we will have to identify acceptable alternative manufacturers. The use of a new manufacturer may cause significant interruptions in supply if the new manufacturer has difficulty manufacturing products to our specifications. Further, the introduction of a new manufacturer may increase the variation in the quality of our products.
If we fail to secure adequate intellectual property protection or become involved in an intellectual property dispute, it could significantly harm our financial results and ability to compete.
Our success will depend, in part, on our ability to obtain and defend patents and protect trade secrets. As of December 31, 2007, sixteen United States patents have been issued to Sonus. A composition of matter patent covering SN2310 issued in the United States in 2007, and national stage applications have been filed in key countries. Nine patents pertaining to our proprietary TOCOSOL technology have issued in the U.S., and TOCOSOL-related patents have also issued in Europe, Canada, Taiwan, Mexico, Korea, and India. Additional patent applications covering our research programs are pending in the U.S. and other countries.
The patent position of medical and pharmaceutical companies is highly uncertain and involves complex legal and factual questions. There can be no assurance that any claims which are included in pending or future patent applications will be issued, that any issued patents will provide us with competitive advantages or will not be challenged by third parties, or that the existing or future patents of third parties will not have an adverse effect on our ability to commercialize our products. Furthermore, there can be no assurance that other companies will not independently develop similar products, duplicate any of our products or design around patents that may be issued to us. Litigation may be necessary to enforce any patents issued to us or to determine the scope and validity of others' proprietary rights in court or administrative proceedings. Any litigation or administrative proceeding could result in substantial costs to us and distraction of our management. An adverse ruling in any litigation or administrative proceeding could have a material adverse effect on our business, financial condition and results of operations.
Our commercial success will depend in part on not infringing patents issued to competitors.
There can be no assurance that patents belonging to competitors will not require us to alter our products or processes, pay licensing fees or cease development of our current or future products. Any
14
litigation regarding infringement could result in substantial costs to us and distraction of our management, and any adverse ruling in any litigation could have a material adverse effect on our business, financial condition and results of operations. Further, there can be no assurance that we will be able to license other technology that we may require at a reasonable cost or at all. Failure by us to obtain a license to any technology that we may require to commercialize our products could have a material adverse effect on our business, financial condition and results of operations. In addition, to determine the priority of inventions and the ultimate ownership of patents, we may participate in interference, reissue or re-examination proceedings conducted by the U.S. Patent and Trademark Office or in proceedings before international agencies with respect to any of our existing patents or patent applications or any future patents or applications, any of which could result in loss of ownership of existing, issued patents, substantial costs to us and distraction of our management.
If we encounter difficulties enrolling patients in our clinical trials, our trials could be delayed or otherwise adversely affected.
Clinical trials for our drug candidates require that we identify and enroll patients with the disorder or condition under investigation. We may not be able to enroll a sufficient number of patients to complete our clinical trials in a timely manner.
Patient enrollment is affected by factors including:
-
- design
of the protocol;
-
- the
size of the patient population;
-
- eligibility
criteria for the study in question;
-
- perceived
risks and benefits of the drug under study;
-
- Institutional
Review Boards/Ethics Committees approvals to conduct the study;
-
- availability
of competing therapies;
-
- efforts
to facilitate timely enrollment in clinical trials;
-
- the
success of our personnel in making the arrangements with potential clinical trial sites necessary for those sites to begin enrolling patients;
-
- patient
referral practices of physicians;
-
- availability
of clinical trial sites; and
-
- other clinical trials seeking to enroll subjects with similar profiles.
If we have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing or planned clinical trials, either of which would have a negative effect on our business.
Reimbursement procedures and future healthcare reform measures are uncertain and may adversely impact our ability to successfully sell pharmaceutical products.
Our ability to successfully sell any pharmaceutical products will depend in part on the extent to which government health administration authorities, private health insurers and other organizations will reimburse patients for the costs of future pharmaceutical products and related treatments. In the United States, government and other third-party payors have sought to contain healthcare costs by limiting both coverage and the level of reimbursement for new pharmaceutical products approved for marketing by the FDA. In some cases, these payors may refuse to provide any coverage for uses of approved products to treat medical conditions even though the FDA has granted marketing approval. Healthcare reform may increase these cost containment efforts. We believe that managed care
15
organizations may seek to restrict the use of new products, delay authorization to use new products or limit coverage and the level of reimbursement for new products. Internationally, where national healthcare systems are prevalent, little if any funding may be available for new products, and cost containment and cost reduction efforts can be more pronounced than in the United States.
If our products are not accepted by the medical community our business will suffer.
Commercial sales of our proposed products will substantially depend upon the products' efficacy and on their acceptance by the medical community. Widespread acceptance of our products will require educating the medical community as to the benefits and reliability of the products. Our proposed products may not be accepted, and, even if accepted, we are unable to estimate the length of time it would take to gain such acceptance.
The businesses in which we engage have a risk of product liability, and in the event of a successful suit against us, our business could be severely harmed.
The testing, marketing and sale of pharmaceutical products entails a risk of product liability claims by consumers and others. We currently maintain product liability insurance for our clinical trials with limits of $10 million per claim and in the aggregate, which we believe to be adequate for current non-commercial and clinical applications of our products. In the event of a successful suit against us, the lack or insufficiency of insurance coverage could have a material adverse effect on our business and financial condition.
Since we use hazardous materials in our business, we may be subject to claims relating to improper handling, storage or disposal of these materials.
Our research and development activities involve the controlled use of hazardous materials, chemicals and various radioactive compounds. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be eliminated completely. In the event of such an accident, we could be held liable for any damages that result and any such liability not covered by insurance could exceed our resources. Compliance with environmental laws and regulations may be expensive, and current or future environmental regulations may impair our research, development or production efforts.
Market volatility may affect our stock price and the value of an investment in our common stock may be subject to sudden decreases.
The trading price for our common stock has been, and we expect it to continue to be, volatile. The price at which our common stock trades depends upon a number of factors, including our historical and anticipated operating results, preclinical and clinical trial results, market perception of the prospects for biotechnology companies as an industry sector and general market and economic conditions, some of which are beyond our control. Factors such as fluctuations in our financial and operating results, changes in government regulations affecting product approvals, reimbursement or other aspects of our or our competitors' businesses, FDA review of our product development activities, the results of preclinical studies and clinical trials, announcements of technological innovations or new commercial products by us or our competitors, developments concerning key personnel and our intellectual property rights, significant collaborations or strategic alliances and publicity regarding actual or potential performance of products under development by us or our competitors could also cause the market price of our common stock to fluctuate substantially. In addition, the stock market has from time to time experienced extreme price and volume fluctuations. These broad market fluctuations may lower the market price of our common stock. Moreover, during periods of stock market price volatility, share prices of many biotechnology companies have often fluctuated in a manner not necessarily
16
related to the companies' operating performance. Also, biotechnology or pharmaceutical stocks may be volatile even during periods of relative market stability. Accordingly, our common stock may be subject to greater price volatility than the stock market as a whole.
We may face fluctuations in operating results.
Our operating results may rise or fall significantly from period to period as a result of many factors, including:
-
- the
amount of research and development we engage in;
-
- outcome
related to strategic activities currently being evaluated;
-
- the
number of product candidates we have, their progress in research, preclinical and clinical studies and the costs involved in manufacturing them;
-
- our
ability to enter into new strategic relationships;
-
- our
ability to maintain our facilities to support our operations;
-
- the
costs involved in prosecuting, maintaining and enforcing patent claims;
-
- the
possibility that others may have or obtain patent rights that are superior to ours;
-
- changes
in government regulation;
-
- changes
in the price of our common stock or other variables used as a basis for valuing stock-based awards;
-
- changes
in accounting policies or principles; and
-
- release of successful products into the market by our competitors.
As a result, we may experience fluctuations in our operating results from quarter to quarter and continue to generate losses. Quarterly comparisons of our financial results may not necessarily be meaningful, and investors should not rely upon such results as an indication of our future performance. In addition, investors may react adversely if our reported operating results are less favorable than in a prior period or are less favorable than those anticipated by investors or the financial community, which may result in a drop in the market price of our common stock.
The impact of the recall by Bristol-Myers Squibb Pharmaceuticals of certain batches of Taxol.
In March 2007, Bristol-Myers Squibb Pharmaceuticals recalled certain batches of Taxol due to potential lack of sterility assurance. At the time of the recall, there had been no reports of non-sterile product and no stability failures had been detected. Among the recalled batches were those being used in the reference arm of the Phase 3 TOCOSOL Paclitaxel pivotal study. Based on the available information, Sonus has no reason to believe that the recalled batches had an adverse impact on patients treated with those batches in the Phase 3 study.
The Company has returned all of the recalled material to its suppliers in accordance with the recall notice. On March 12, 2008, the Company received an initial refund from its suppliers of approximately $850,000 for returned material. While we believe that we will receive a refund for the remaining returned material, we are not able to reasonably estimate an amount of the refund at this time, and there can be no assurance that we will receive a full refund or that it will be received on a timely basis.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
17
During 2007 the Company occupied approximately 27,000 square feet of laboratory and office space in a single facility near Seattle, Washington. The lease on this facility expired in July 2007, and was extended through December 31, 2007. In November 2006 the Company signed a lease agreement for a larger facility also near Seattle, Washington. The Company moved into this facility on December 14, 2007. The new lease involves approximately 42,600 square feet of laboratory and office space in a single facility. The lease has a 10 year term and includes two options to renew for additional 5 year periods. This facility is expected to be sufficient to meet the Company's current and anticipated requirements throughout the term of the lease.
From time to time, the Company may be involved in litigation relating to claims arising out of our operations in the normal course of business. The Company currently is not a party to any legal proceedings, the adverse outcome of which, in management's opinion, individually or in the aggregate, would have a material adverse effect on the Company's results of operations or financial position.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted to a vote of security holders during the fourth quarter of the year ended December 31, 2007.
ITEM 5. MARKET FOR THE REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock first began trading on the Nasdaq National Market under the symbol SNUS on October 12, 1995. No cash dividends have been paid on the common stock, and we do not anticipate paying any cash dividends in the foreseeable future. As of March 3, 2008, there were approximately 160 stockholders of record and approximately 8,450 beneficial stockholders of our Common Stock. The high and low sales prices of our common stock as reported by Nasdaq Global Market (formerly the NASDAQ National Market) for the periods indicated are as follows:
|
|
High |
Low |
||||
|---|---|---|---|---|---|---|
| 2007 | ||||||
| First Quarter | $ | 6.22 | $ | 4.55 | ||
| Second Quarter | 6.25 | 4.91 | ||||
| Third Quarter | 5.43 | .59 | ||||
| Fourth Quarter | .70 | .40 | ||||
|
2006 |
||||||
| First Quarter | $ | 6.92 | $ | 4.85 | ||
| Second Quarter | 6.28 | 4.40 | ||||
| Third Quarter | 5.15 | 4.25 | ||||
| Fourth Quarter | 6.32 | 4.51 | ||||
The information required by this item regarding equity compensation plan information is set forth in Part III, Item 12 of this Annual Report filed on Form 10-K. We made no purchases of equity securities during the fourth quarter of the year ended December 31, 2007.
18
Stock Performance Graph
Set forth below is a line graph comparing the cumulative stockholder return on the Company's Common Stock with the cumulative total return of the Nasdaq Composite Index and the Nasdaq Pharmaceutical Index for the five year period that commenced December 31, 2001 and ended on December 31, 2007.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among SONUS Pharmaceuticals, Inc., The NASDAQ Composite Index
And The NASDAQ Pharmaceutical Index
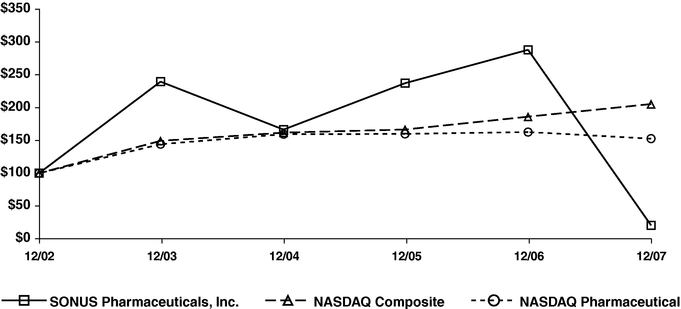
- *
- $100 invested on 12/31/02 in stock or index-including reinvestment of dividends. Fiscal year ending December 31.
19
ITEM 6. SELECTED FINANCIAL DATA
The data set forth below should be read in conjunction with Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the Financial Statements and Notes thereto appearing at Item 8 of this report.
|
|
Year Ended December 31, |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
2007 |
2006 |
2005 |
2004 |
2003 |
|||||||||||||
|
|
(in thousands, except per share data) |
|||||||||||||||||
| Statements of Operations Data: | ||||||||||||||||||
| Total revenue | $ | 20,131 | $ | 22,392 | $ | 8,254 | $ | | $ | 25 | ||||||||
| Operating expenses | $ | 35,366 | $ | 48,679 | $ | 30,064 | $ | 16,576 | $ | 10,663 | ||||||||
| Net loss | $ | (13,063 | ) | $ | (23,551 | ) | $ | (21,097 | ) | $ | (16,311 | ) | $ | (10,467 | ) | |||
| Net loss per share: | ||||||||||||||||||
| Basic | $ | (0.35 | ) | $ | (0.68 | ) | $ | (0.88 | ) | $ | (0.81 | ) | $ | (0.68 | ) | |||
| Diluted | $ | (0.35 | ) | $ | (0.68 | ) | $ | (0.88 | ) | $ | (0.81 | ) | $ | (0.68 | ) | |||
| Shares used in calculation of net loss per share | ||||||||||||||||||
| Basic | 36,909 | 34,730 | 24,027 | 20,169 | 15,504 | |||||||||||||
| Diluted | 36,909 | 34,730 | 24,027 | 20,169 | 15,504 | |||||||||||||
|
|
December 31, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
2007 |
2006 |
2005 |
2004 |
2003 |
|||||||||||
|
|
(in thousands) |
|||||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| Cash, cash equivalents and marketable securities | $ | 34,199 | $ | 58,278 | $ | 49,318 | $ | 20,580 | $ | 19,664 | ||||||
| Accounts receivable from Bayer Schering Pharma AG | $ | | $ | 8,044 | $ | 7,057 | $ | | $ | | ||||||
| Total assets | $ | 45,249 | $ | 68,493 | $ | 57,914 | $ | 22,571 | $ | 21,468 | ||||||
| Current liabilities | $ | 6,369 | $ | 19,910 | $ | 11,242 | $ | 3,255 | $ | 1,794 | ||||||
| Long-term liabilities | $ | 6,976 | $ | 5,541 | $ | 11,408 | $ | 239 | $ | 364 | ||||||
| Stockholders' equity | $ | 31,904 | $ | 43,042 | $ | 35,264 | $ | 19,077 | $ | 19,310 | ||||||
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward-Looking Statements
This report contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and we intend that such forward-looking statements be subject to the safe harbors created thereby. Examples of these forward-looking statements include, but are not limited to:
-
- timing
and amount of future contractual payments, product revenue and operating expenses;
-
- progress
and preliminary results of clinical trials;
-
- our
anticipated future capital requirements and the terms of any capital financing agreements;
-
- anticipated
regulatory filings, requirements and future clinical trials; and
-
- market acceptance of our products and the estimated potential size of these markets.
While these forward-looking statements made by us are based on our current beliefs and judgments, they are subject to risks and uncertainties that could cause actual results to vary from the projections in the forward-looking statements. You should consider the risks below carefully in addition
20
to other information contained in this report before engaging in any transaction involving shares of our common stock. If any of these risks occur, they could seriously harm our business, financial condition or results of operations. In such case, the trading price of our common stock could decline, and you may lose all or part of your investment.
The discussion and analysis set forth in this document contains trend analysis, discussions of regulatory status and other forward-looking statements. Actual results could differ materially from those projected in the forward-looking statement as a result of the following factors, among others:
-
- future
capital requirements and uncertainty of obtaining additional funding through corporate parterships, debt or equity financings;
-
- ability
to integrate and realize benefits from strategic opportunites, including mergers and acquisitions;
-
- continued
listing on the NASDAQ Global Market (formerly NASDAQ National Market);
-
- results
of research and preclinical studies may not be indicative of results in humans;
-
- ability
to build out our product candidate pipeline through internal development, product in-licensing or acquisition activities;
-
- proper
management of our operations will be critical to the success of the company;
-
- history
of operating losses and uncertainty of future financial results;
-
- volatility
in the value of our common stock;
-
- dependence
on the development and commercialization of products;
-
- uncertainty
of governmental regulatory requirements and lengthy approval process;
-
- dependence
on third parties for funding, clinical development, regulatory approvals, manufacturing and distribution;
-
- dependence
on key employees;
-
- uncertainty
of U.S. or international legislative or administrative actions;
-
- competition
and risk of competitive new products;
-
- limited
manufacturing experience and dependence on a limited number of contract manufacturers and suppliers;
-
- ability
to obtain and defend patents, protect trade secrets and avoid infringing patents held by third parties;
-
- limitations
on third-party reimbursement for medical and pharmaceutical products;
-
- acceptance
of our products by the medical community;
-
- potential
for product liability issues and related litigation;
-
- potential
for claims arising from the use of hazardous materials in our business;
-
- fluctuations
in our operating results; and
-
- uncertainty relating to the timing and results of clinical trials.
21
MD&A Overview
In Management's Discussion and Analysis of Financial Condition and Results of Operations we explain the general financial condition and the results of operations for our Company, including:
-
- An
overview of our business;
-
- Results
of operations and why those results are different from the prior year; and
-
- The capital resources we currently have and possible sources of additional funding for future capital requirements.
Overview
Sonus Pharmaceuticals is developing novel small molecule drugs for the treatment of patients with cancer. Our objective is to identify opportunities where there is the possibility for major improvements in patient treatments and where we believe we can mitigate development risk. We currently have one drug in clinical development and two earlier stage programs. Our plan is to continue to develop our internal pipeline of clinical compound opportunities and to evaluate possible strategic alternatives, including in-licensing, out-licensing and merger and acquisition opportunities, as a means of achieving our business strategies and enhancing stockholder value. In the fourth quarter of 2007 we engaged Ferghana Partners Inc., an international provider of independent financial advisory services to firms in the biotechnology, pharmaceuticals, diagnostics and specialty chemicals industries, to assist us with these strategic alternatives.
Results of Operations
As of December 31, 2007, our accumulated deficit was approximately $124.8 million. We expect to incur substantial additional operating losses over the next several years. Such losses have been and will continue to principally be the result of various costs associated with our discovery and research and development programs. Substantially all of our working capital in recent years has resulted from equity financings and payments under corporate partnership agreements. Our ability to ever achieve a consistent, profitable level of operations depends in large part on obtaining regulatory approval for future product candidates in addition to successfully manufacturing and marketing those products once they are approved. Even if we are successful in the aforementioned activities our operations may not be profitable.
Collaboration and License Agreement with Bayer Schering
On October 17, 2005, Sonus entered into a Collaboration and License Agreement with Bayer Schering Pharma AG, pursuant to which, among other things, we granted Bayer Schering an exclusive, worldwide license to TOCOSOL® Paclitaxel. At that time, the parties agreed to a core development program consisting of the initial pivotal trial in metastatic breast cancer, trials for additional indications and trials to support launch of TOCOSOL Paclitaxel, and agreed to share equally in the costs of the core development program. In connection with the Bayer Agreement, the Company and an affiliate of Bayer Schering entered into a Securities Purchase Agreement whereby the Company sold 3,900,000 shares of common stock for an aggregate of $15.7 million and warrants to purchase 975,000 shares of common stock for an aggregate purchase price of $122,000.
On October 3, 2007, Sonus received notification from Bayer Schering of its decision to terminate the Bayer Agreement in accordance with its terms because the Phase 3 pivotal trial did not meet its primary endpoint and the results of the trial did not support, in Bayer Schering's judgment, a submission for a New Drug Application with the FDA. The termination was effective on November 2, 2007. In accordance with the terms of the Bayer Agreement, all rights to TOCOSOL Paclitaxel have reverted back to Sonus. We have discontinued development of TOCOSOL Paclitaxel due to results of
22
the Phase 3 study and the time and cost that would be required to conduct the necessary clinical studies to continue development, which at a minimum, would include another Phase 3 pivotal trial. These closure activities were substantially complete by December 31, 2007. Due to the termination of the Bayer Agreement by Bayer Schering, in October 2007, the Company recognized $6.9 million of revenue in the fourth quarter of 2007, which represents the balance of the unamortized deferred revenue from the upfront license fee. During 2007, the Company recognized a total of $11.0 million of revenue from amortization of the deferred revenue, and $9.1 million of revenue related primarily to reimbursable Phase 3 activity through the date of termination and expenses associated with the termination of the Phase 3 trial. The Company does not expect recognition of any revenue or expense related to the Bayer Agreement beyond 2007. The final net billing between the Company and Bayer Schering was completed during the fourth quarter of 2007. Settlement of the final amount outstanding was received from Bayer Schering in December 2007. No receivables from or payables to Bayer Schering are outstanding at December 31, 2007.
Years Ended December 31, 2007 and December 31, 2006
Our revenue was $20.1 million for the year ended December 31, 2007 as compared with $22.4 million for 2006. Revenue in 2007 and 2006 was fully attributable to the Bayer Agreement. In 2007 we recognized $11.0 million of revenue in amortization of the upfront license fee, including $6.9 million in the fourth quarter, which represents the balance of the unamortized deferred revenue due to the termination of the Bayer Agreement. An additional $9.1 million in research and development reimbursements were recognized under the terms of the Bayer Agreement in 2007. There was a final net billing to Bayer Schering in the fourth quarter of 2007 for accrued expenses related primarily to reimbursable Phase 3 activity through the date of termination and expenses associated with the termination of the Phase 3 trial. The Company does not expect recognition of any revenue or expense related to the Bayer Agreement beyond 2007.
Our research and development (R&D) expenses were $27.1 million for the year ended December 31, 2007 compared with $40.8 million for 2006. The decrease in 2007 was primarily the result of lower spending on clinical trials and drug supply and manufacturing costs related to the Phase 3 trial for TOCOSOL Paclitaxel, which was terminated in the fourth quarter of 2007. We expect R&D expenses to decrease in 2008 due to the termination of the TOCOSOL Paclitaxel program in 2007.
Our general and administrative (G&A) expenses were $8.2 million for the year ended December 31, 2007 compared with $7.9 million for 2006. The 2007 increase was primarily related to increased market research conducted on TOCOSOL Paclitaxel in the first three quarters prior to the termination of the program, and costs associated with staff reductions in the fourth quarter. We expect G&A expenses to be lower than levels experienced in 2007; however, should we enter into any strategic transaction G&A expenses could be affected.
Our total operating expenses in 2008 are expected to decrease from 2007 levels due to the termination of research and development activities for TOCOSOL Paclitaxel and the reduction of workforce which was effective November 30, 2007. We estimate that R&D spending will comprise approximately 65%-70% of the anticipated spending in 2008. A significant portion of the R&D spending will be devoted to development activities for SN2310 and other compounds in our pipeline. These estimates and actual expenses are subject to change depending on many factors.
Our interest income, net of interest expense, was $2.3 million for the year ended December 31, 2007 compared with $2.8 million for 2006. The 2007 decrease was due primarily to lower levels of invested cash in 2007.
The Company had no income tax expense in 2007, 2006 or 2005 as it had incurred pretax losses.
23
Years Ended December 31, 2006 and December 31, 2005
Our revenue was $22.4 million for the year ended December 31, 2006 as compared with $8.3 million for 2005. Revenue in 2006 and 2005 was fully attributable to the Bayer Agreement. We recognized $5.5 million in amortization of the upfront license fee and an additional $16.9 million in research and development reimbursements under the terms of our agreement with Bayer Schering.
Our research and development (R&D) expenses were $40.8 million for the year ended December 31, 2006 compared with $24.2 million for 2005. The 2006 increase was primarily the result of the spending associated with the Phase 3 clinical trial for TOCOSOL Paclitaxel including both clinical and drug supply and manufacturing costs (both control and study drug) as well as costs associated with the implementation of SFAS 123R.
Our general and administrative (G&A) expenses were $7.9 million for the year ended December 31, 2006 compared with $5.9 million for 2005. The 2006 increase was primarily attributed to costs associated with the implementation of SFAS 123R as well as market research conducted on TOCOSOL Paclitaxel as that product moved closer to FDA submission.
Our interest income, net of interest expense, was $2.8 million for the year ended December 31, 2006 compared with $708,000 for 2005. The 2006 increase was due primarily to higher levels of invested cash in 2006 in addition to generally higher interest rates throughout 2006.
The Company had no income tax expense in 2006, 2005 or 2004 as it had incurred pretax losses.
Liquidity and Capital Resources
We have historically financed operations with proceeds from equity financings and payments under collaboration agreements with third parties. At December 31, 2007, we had cash, cash equivalents and marketable securities totaling $34.2 million compared to $58.3 million at December 31, 2006. The decrease was primarily due to the net loss of $13.1 million, which includes $11.0 million of revenue recognized in 2007 from amortization of the upfront license fee received by Sonus in prior years, in addition to timing of items accrued in 2006 and paid in 2007.
Net cash used in operating activities for the years ended December 31, 2007, 2006 and 2005, was $23.3 million, $19.7 million and $8.4 million, respectively. Expenditures in all periods were primarily a result of R&D expenses, including clinical trial costs, and G&A expenses in support of our operations and product development activities. R&D expenses were primarily related to TOCOSOL Paclitaxel and to a lesser extent other potential product candidates. We believe that G&A expenses will decrease in 2008 due to staff reductions which were effective in the fourth quarter of 2007. We expect R&D expenses to decrease in 2008 due to the termination of the TOCOSOL Paclitaxel program in 2007. We recorded $20.1 million in revenue in 2007, $22.4 million in 2006 and $8.3 million in 2005, all of which was fully attributable to the Bayer Agreement. We expect no revenue or expense related to the Bayer Agreement beyond 2007. We paid no corporate income taxes in any of the periods presented.
Net cash provided by (used in) investing activities for the years ended December 31, 2007, 2006 and 2005, was ($6.1) million, ($23.0) million and $20.1 million, respectively. The net cash used in investing activities during 2007 and 2006 was primarily due to transactions involving marketable securities in the normal course of business in addition to purchases of fixed assets. Activity related to marketable securities relates primarily to the investment of money raised in equity financings or received under the Bayer Agreement. The related maturities and sales of those investments provide us with working capital on an as-needed basis. We also initiate shifts between cash equivalent securities and marketable securities based on our cash needs and the prevailing interest rate environment.
24
Net cash provided by financing activities for the years ended December 31, 2007, 2006 and 2005, was $0.2 million, $29.1 million and $37.2 million, respectively. The net cash provided by financing activities in 2007 was primarily due to the issuance of common stock under employee benefit plans and the exercise of common stock warrants.The net cash provided by financing activities in 2006 and 2005 primarily related to proceeds from equity financing, the exercise of common stock warrants and the issuance of common stock under employee benefit plans.
We expect that our cash requirements will decrease in 2008 due to the termination of development of TOCOSOL Paclitaxel and related staff reductions. Under our current forecasted cash needs, which assume continued development of SN2310 and other earlier stage product candidates, we believe that existing cash, cash equivalents and marketable securities will be sufficient to fund expected operations into the third quarter of 2009. We will need additional capital in 2009 to support the continued development SN2310, other product candidates and to fund continuing operations. Our future capital requirements depend on many factors including:
-
- our
ability to obtain and timing of payments under equity or debt financings;
-
- outcome
related to strategic activities currently being evaluated;
-
- timing
and costs of preclinical development, clinical trials and regulatory approvals;
-
- timing
and cost of drug discovery and research and development;
-
- entering
into new collaborative or product license agreements for products in our pipeline; and
-
- costs related to obtaining, defending and enforcing patents.
We have contractual obligations in the form of operating leases which expire between 2010 and 2017. We signed a new facility lease in November 2006. The new facility lease has a term of 10 years with a provision for two additional five year renewals. The term commencement date for the new lease is January 1, 2008. The following table summarizes our contractual obligations under these agreements as of December 31, 2007:
|
Contractual Obligations |
Total |
Less than 1 year |
1-3 years |
3-5 years |
More than 5 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Operating lease obligations | $ | 21,655,816 | $ | 1,929,120 | $ | 3,979,216 | $ | 4,171,944 | $ | 11,575,536 | |||||
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements and have not entered into any transactions involving unconsolidated, limited purpose entities.
Lease Agreement
In November 2006 the Company entered into a new operating lease agreement for combined laboratory and office space. Our previous operating lease for facilities expired December 31, 2007, and we moved into the newly leased facility in December 2007. The new lease, as amended in 2007, is for approximately 42,600 square feet and expires on December 31, 2017, with a provision for two additional five year renewals. In connection with the new lease, we received landlord-provided incentives of approximately $7.7 million in the form of tenant improvements, which have been recorded as additions to fixed assets and deferred rent liabilities and will be amortized over the inital ten year term of the lease. In connection with our new lease arrangement, we were required to provide a cash security deposit of approximately $497,000 of which approximately $440,000 was paid upon lease signing in November 2006, and the remainder was paid in February 2008. In addition, the lease stipulates the Company must issue a standby letter of credit for approximately $500,000 which is expected to be issued early in 2008.
25
Critical Accounting Policies and Estimates
We prepare our financial statements in conformity with accounting principles generally accepted in the United States of America. As such, we are required to make certain estimates, judgments and assumptions that we believe are reasonable based upon the information available. These estimates and assumptions affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the periods presented. Actual results could differ significantly from those estimates under different assumptions and conditions. We believe that the following discussion addresses our most critical accounting estimates which are those that are most important to the portrayal of our financial condition and results of operations and which require our most difficult and subjective judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. We also have other policies that we consider key accounting policies; however, these policies do not meet the definition of critical accounting estimates, because they do not generally require us to make estimates or judgments which are difficult or subjective.
-
-
Cash, Cash Equivalents and Marketable Securities. We consider investments in highly liquid instruments purchased
with a remaining maturity at purchase of 90 days or less to be cash equivalents. Investments with a remaining maturity at purchase in excess of 90 days are classified as marketable
securities. The amounts are recorded at cost, which approximate fair market value. Our cash equivalents and marketable securities consist principally of commercial paper, money market securities,
repurchase agreements, corporate bonds/notes and government agency securities. We have classified our entire investment portfolio as available-for-sale.
Available-for-sale securities are carried at fair value, with unrealized gains and losses reported as a separate component of stockholders' equity and included in accumulated
other comprehensive income. The amortized cost of investments is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion are included in interest
income. Interest earned on securities is included in interest income. We consider marketable securities with maturity greater than twelve months long-term and maturity less than twelve
months short-term.
-
-
Revenue Recognition. Since inception, we have generated revenue from collaborative agreements, licensing fees
and from the assignment of developed and patented technology. These arrangements may include upfront non-refundable payments, development milestone payments, payments for research and
development services performed and product sales royalties or revenue. Our revenue recognition policies are based on the requirements of SEC Staff Accounting Bulletin No. 104
"Revenue Recognition," and, for contracts with multiple deliverables, we allocate arrangement consideration based on the fair value of the elements
under guidance from Emerging Issues Task Force Issue 00-21 ("EITF 00-21"), "Revenue Arrangements with Multiple
Deliverables." Under EITF 00-21, revenue arrangements with multiple deliverables are divided into separate units of accounting and revenue is allocated to
these units based upon relative fair values with revenue recognition criteria considered separately for each unit.
Nonrefundable upfront technology license fees, for product candidates where we are providing continuing services related to product development, are deferred and recognized as revenue over the estimated development period.
The timing and amount of revenue that we recognize from licenses of technology, either from upfront fees or milestones where we are providing continuing services related to product development, is primarily dependent upon our estimates of the development period. We define the development period as the point from which research activities commence up to FDA approval of our submission assuming no further research is necessary. As product candidates move through the development process, it is necessary to revise these estimates to consider changes to the product development cycle, such as changes in the clinical development plan,
26
-
-
Research and Development Expenses. Pursuant to SFAS No. 2 "Accounting for
Research and Development Costs," our research and development costs are expensed as incurred. Research and development expenses include, but are not limited to, payroll and
personnel expenses, lab expenses, clinical trial and related clinical manufacturing costs, facilities and overhead costs. Clinical trial expenses, which are included in research and development
expenses and represent a significant portion
of our research and development expenditures, represent obligations resulting from our contracts with various clinical research organizations in connection with conducting clinical trials for our
product candidates. We recognize expenses for these contracted activities based on a variety of factors, including actual and estimated labor hours, clinical site initiation activities, patient
enrollment rates, estimates of external costs and other activity-based factors. We believe that this method best approximates the efforts expended on a clinical trial with the expenses we record. We
adjust clinical expense estimates when actual results are available.
-
-
Stock-based Compensation. We adopted the requirements of SFAS 123R, "Share-Based
Payment," effective January 1, 2006, utilizing the "modified prospective" method. We use the Black-Scholes-Merton option pricing model as the most appropriate
fair-value method for our awards and recognize compensation cost on a straight-line basis over our awards' vesting periods in accordance with the provisions of
SFAS 123R. In valuing our options using the Black-Scholes-Merton option pricing model, we make assumptions about risk-free interest rates, dividend yields, volatility and weighted
average expected lives of the options. Risk-free interest rates are derived from United States treasury securities as of the option grant date. Dividend yields are based on our historical
dividend payments, which have been zero to date. Volatility is derived from the historical volatility of our common stock as traded on NASDAQ. Forfeiture rates are estimated using historical actuals
over the estimated life of the option grant. These rates are adjusted on a quarterly basis and any change in compensation expense is recognized in the period of the change. The weighted average
expected lives of the options is based on historical experience of option exercises and the average vesting option schedule. For unvested awards granted prior to the adoption date, compensation
expense is based on the original grant date fair value measurement under SFAS 123. We currently believe that the assumptions used to generate those fair values are appropriate.
-
-
Income Taxes. Effective January 1, 2007, the Company adopted the provisions of the Financial
Interpretation No. 48, "Accounting for Uncertainty in Income Taxesan interpretation of FASB Statement No. 109"
("FIN 48"). FIN 48 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken
in a tax return. At the date of adoption of FIN 48, we had no unrecognized tax benefits and expected no significant changes in unrecognized tax benefits in the next twelve months. The adoption
of this statement did not result in a cumulative accounting adjustment and did not impact our financial position, results of operations or cash flows.
We recognize interest and penalties related to uncertain tax positions in income tax expense when applicable. To date, there have been no interest or penalties charged to the Company in relation to the underpayment of income taxes. Due to the uncertainty of ultimately realizing tax benefits, we record a valuation allowance equal to our total net deferred tax assets.
regulatory requirements, or various other factors, many of which may be outside of our control. Should our clinical development plans change, as a result of regulatory or other matters, we would adjust our development period estimates accordingly. The impact on revenue of changes in our estimates and the timing thereof is recognized prospectively over the remaining estimated product development period. Revenue from research and development services performed under collaboration agreements is generally recognized in the period when the services are performed. Payments received in excess of amounts earned are recorded as deferred revenue.
27
Recent Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board ("FASB") issued SFAS No. 157, "Fair Value Measurements," which establishes a framework for measuring fair value, and expands disclosures about fair value measurements. This statement does not require any new fair value measurements, but increases consistency and comparability in the use of fair value measurements and calculations. This statement is effective for financial statements issued for fiscal years beginning after November 15, 2007 and for interim periods within those fiscal years. Management does not anticipate that the adoption of SFAS No. 157 will have a material effect on our financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159, "The Fair Value Option for Financial Assets and Financial LiabilitiesIncluding an amendment to FASB Statement No. 115". SFAS 159 allows companies to choose to measure eligible assets and liabilities at fair value with changes in value recognized in earnings. Fair value treatment for eligible assets and liabilities may be elected either prospectively upon initial recognition, or if an event triggers a new basis of accounting for an existing asset or liability. SFAS 159 is effective in the first quarter of 2008, and the Company is currently evaluating the impact of adoption on its financial position and results of operations.
In June 2007, the EITF reached a consensus on EITF No. 07-03, Accounting for Nonrefundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities, or EITF 07-03. EITF 07-03 requires that nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities be deferred and capitalized and recognized as an expense as the goods are delivered or the related services are performed. EITF 07-03 is effective for fiscal years beginning after December 15, 2007, and will be adopted by the Company in the first quarter of 2008. The adoption of EITF 07-3 will have the effect of changing our policy on nonrefundable prepayments for research and development services whereby such costs will be deferred and recognized as the services are rendered as compared to the existing policy whereby such payments are charged to research and development expense as paid. This change may have an impact on financial condition and the results of operations in future periods.
In December 2007, the EITF reached a consensus on EITF No. 07-01, Accounting for Collaborative Arrangements Related to the Development and Commercialization of Intellectual Property, or EITF 07-01. EITF 07-01 discusses the appropriate income statement presentation and classification for the activities and payments between the participants in arrangements related to the development and commercialization of intellectual property. The sufficiency of disclosure related to these arrangements is also specified. EITF 07-01 is effective for fiscal years beginning after December 15, 2008. As a result, EITF 07-01 is effective for us in the first quarter of fiscal 2009. We do not expect the adoption of EITF 07-01 to have a material impact on either our financial position or results of operations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest rate risk:
The market risk inherent in our marketable securities portfolio represents the potential loss arising from adverse changes in interest rates. If market rates hypothetically increase immediately and uniformly by 100 basis points from levels at December 31, 2007, the decline in the fair value of the investment portfolio would not be material. Given the short-term nature of our investment portfolio, we do not expect our operating results or cash flows to be affected to any significant degree by a sudden change in market interest rates.
28
Foreign currency exchange risk:
We are exposed to risks associated with foreign currency transactions on certain contracts denominated in foreign currencies (primarily Euro and Pound Sterling denominated contracts) and we have not hedged these amounts. As our unhedged foreign currency transactions fluctuate, our earnings might be negatively affected. Accordingly, changes in the value of the U.S. dollar relative to the Euro/Pound Sterling might have an adverse effect on our reported results of operations and financial condition, and fluctuations in exchange rates might harm our reported results and accounts from period to period. The impact of foreign currency fluctuations related to realized gains and losses during the past three years has not been material.
29
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
INDEX TO FINANCIAL STATEMENTS: |
|
|
|---|---|---|
| Report of Independent Registered Public Accounting Firm | 31 | |
Balance Sheets as of December 31, 2007 and 2006 |
32 |
|
Statements of Operations for the years ended December 31, 2007, 2006, and 2005 |
33 |
|
Statements of Stockholders' Equity for the years ended December 31, 2007, 2006, and 2005 |
34 |
|
Statements of Cash Flows for the years ended December 31, 2007, 2006, and 2005 |
35 |
|
Notes to the Financial Statements |
36 |
30
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders
Sonus Pharmaceuticals, Inc.
We have audited the accompanying balance sheets of Sonus Pharmaceuticals, Inc. (the Company) as of December 31, 2007 and 2006, and the related statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2007. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Sonus Pharmaceuticals, Inc. at December 31, 2007 and 2006, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2007, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Sonus Pharmaceuticals, Inc.'s internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 14, 2008 expressed an unqualified opinion thereon.
As discussed in Note 8 to the consolidated financial statements, in 2007 the Company changed its accounting for income taxes upon the adoption of Financial Accounting Standard Board Interpretation No. 48 Accounting for Uncertainty in Income Taxes an interpretation of FASB Statement No.109 and as discussed in Note 9 to the consolidated financial statements, in 2006 the Company changed its method of accounting for stock-based compensation upon the adoption of Statement of Financial Accounting Standards No. 123R Share-Based Payment, effective January 1, 2006.
| ERNST & YOUNG LLP | ||
Seattle, Washington March 14, 2008 |
31
Sonus Pharmaceuticals, Inc.
Balance Sheets
|
|
December 31, |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
2007 |
2006 |
|||||||
| ASSETS | |||||||||
| Current assets: | |||||||||
| Cash and cash equivalents | $ | 6,535,272 | $ | 35,771,784 | |||||
| Marketable securities | 27,663,554 | 22,506,086 | |||||||
| Accounts receivable from Bayer Schering Pharma AG | | 8,043,771 | |||||||
| Interest receivable | 456,149 | 79,439 | |||||||
| Other current assets | 576,905 | 445,031 | |||||||
| Total current assets | 35,231,880 | 66,846,111 | |||||||
Equipment, furniture and leasehold improvements, net |
9,577,567 |
1,186,174 |
|||||||
| Other assets | 439,822 | 460,717 | |||||||
Total assets |
$ |
45,249,269 |
$ |
68,493,002 |
|||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||||
| Current liabilities: | |||||||||
| Accounts payable | $ | 1,462,444 | $ | 898,486 | |||||
| Accounts payable to Bayer Schering Pharma AG | | 1,473,050 | |||||||
| Accrued expenses | 4,141,273 | 11,928,124 | |||||||
| Deferred revenue from Bayer Schering Pharma AG | | 5,545,919 | |||||||
| Current portion of deferred rent | 765,005 | | |||||||
| Other current liabilities | | 64,792 | |||||||
| Total current liabilities | 6,368,722 | 19,910,371 | |||||||
Deferred revenue from Bayer Schering Pharma AG, less current portion |
|
5,540,694 |
|||||||
| Deferred rent, less current portion | 6,976,130 | | |||||||
Commitments and contingencies |
|||||||||
| Stockholders' equity: | |||||||||
| Preferred stock, $.001 par value: | |||||||||
| 5,000,000 shares authorized; no shares outstanding | | | |||||||
| Common stock, $.001 par value: | |||||||||
| 75,000,000 shares authorized; 37,048,335 and 36,853,974 shares issued and outstanding in 2007 and 2006, respectively | 156,704,899 | 154,780,939 | |||||||
| Accumulated deficit | (124,801,837 | ) | (111,738,669 | ) | |||||
| Accumulated other comprehensive income (loss) | 1,355 | (333 | ) | ||||||
| Total stockholders' equity | 31,904,417 | 43,041,937 | |||||||
Total liabilities and stockholders' equity |
$ |
45,249,269 |
$ |
68,493,002 |
|||||
See accompanying notes.
32
Sonus Pharmaceuticals, Inc.
Statements of Operations
|
|
Year Ended December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
2007 |
2006 |
2005 |
|||||||||
| Revenue: | ||||||||||||
| Collaboration revenue from Bayer Schering Pharma AG | $ | 20,130,663 | $ | 22,391,858 | $ | 8,254,483 | ||||||
Operating expenses: |
||||||||||||
| Research and development | 27,146,725 | 40,795,790 | 24,209,152 | |||||||||
| General and administrative | 8,218,890 | 7,882,762 | 5,854,550 | |||||||||
| Total operating expenses | 35,365,615 | 48,678,552 | 30,063,702 | |||||||||
Operating loss |
(15,234,952 |
) |
(26,286,694 |
) |
(21,809,219 |
) |
||||||
Other income (expense): |
||||||||||||
| Other (expense) income | (125,351 | ) | (112,490 | ) | 4,160 | |||||||
| Interest income | 2,297,569 | 2,850,872 | 714,866 | |||||||||
| Interest expense | (434 | ) | (2,984 | ) | (6,824 | ) | ||||||
| Total other income, net | 2,171,784 | 2,735,398 | 712,202 | |||||||||
Net loss |
$ |
(13,063,168 |
) |
$ |
(23,551,296 |
) |
$ |
(21,097,017 |
) |
|||
Basic and diluted net loss per share |
$ |
(0.35 |
) |
$ |
(0.68 |
) |
$ |
(0.88 |
) |
|||
Shares used in calculation of basic and diluted net loss per share |
36,909,462 |
34,729,930 |
24,027,127 |
|||||||||
See accompanying notes.
33
Sonus Pharmaceuticals, Inc.
Statements of Stockholders' Equity
|
|
Common Stock |
|
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
Accumulated Other Comprehensive Income (Loss) |
|
|||||||||||||
|
|
Shares |
Amount |
Accumulated Deficit |
Total |
||||||||||||
| Balance at January 1, 2005 | 21,352,795 | 86,202,180 | (67,090,356 | ) | (34,643 | ) | 19,077,181 | |||||||||
| Comprehensive income (loss): | ||||||||||||||||
| Net loss | | | (21,097,017 | ) | | (21,097,017 | ) | |||||||||
| Change in unrealized gain (loss) on investments | | | | 42,142 | 42,142 | |||||||||||
| Comprehensive loss | (21,054,875 | ) | ||||||||||||||
| Issuance of common stock under employee benefit plans | 86,082 | 185,117 | | | 185,117 | |||||||||||
| Exercise of common stock warrants | 575,000 | 2,351,750 | | | 2,351,750 | |||||||||||
| Issuance of common stock and common stock warrants (net of offering costs of $1,180,669) | 8,551,869 | 34,704,619 | | | 34,704,619 | |||||||||||
| Balance at December 31, 2005 | 30,565,746 | 123,443,666 | (88,187,373 | ) | 7,499 | 35,263,792 | ||||||||||
| Comprehensive income (loss): | ||||||||||||||||
| Net loss | | | (23,551,296 | ) | | (23,551,296 | ) | |||||||||
| Change in unrealized gain (loss) on investments | | | | (7,832 | ) | (7,832 | ) | |||||||||
| Comprehensive loss | (23,559,128 | ) | ||||||||||||||
| Issuance of common stock under employee benefit plans | 84,553 | 292,113 | | | 292,113 | |||||||||||
| Stock-based compensation expense | | 2,173,379 | | | 2,173,379 | |||||||||||
| Exercise of common stock warrants | 73,675 | 301,330 | | | 301,330 | |||||||||||
| Issuance of common stock (net of offering costs of $2,079,549) | 6,130,000 | 28,570,451 | | | 28,570,451 | |||||||||||
| Balance at December 31, 2006 | 36,853,974 | $ | 154,780,939 | $ | (111,738,669 | ) | $ | (333 | ) | $ | 43,041,937 | |||||
| Comprehensive income (loss): | ||||||||||||||||
| Net loss | | | (13,063,168 | ) | | (13,063,168 | ) | |||||||||
| Change in unrealized gain (loss) on investments | | | | 1,688 | 1,688 | |||||||||||
| Comprehensive loss | (13,061,480 | ) | ||||||||||||||
| Issuance of common stock under employee benefit plans | 179,317 | 205,325 | | | 205,325 | |||||||||||
| Stock-based compensation expense | | 1,661,195 | | | 1,661,195 | |||||||||||
| Exercise of common stock warrants | 14,044 | 57,440 | | | 57,440 | |||||||||||
| Balance at December 31, 2007 | 37,047,335 | $ | 156,704,899 | $ | (124,801,837 | ) | $ | 1,355 | $ | 31,904,417 | ||||||
See accompanying notes.
34
Sonus Pharmaceuticals, Inc.
Statements of Cash Flows
|
|
Year Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
2007 |
2006 |
2005 |
||||||||
| Operating activities: | |||||||||||
| Net loss | $ | (13,063,168 | ) | $ | (23,551,296 | ) | $ | (21,097,017 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
| Depreciation and amortization | 655,689 | 609,615 | 592,825 | ||||||||
| Non-cash stock-based compensation | 1,661,195 | 2,173,379 | | ||||||||
| Accretion of net discount on securities | (402,655 | ) | (301,312 | ) | (6,669 | ) | |||||
| (Gain) loss on sale of capital equipment | 22,015 | | (4,160 | ) | |||||||
| Changes in operating assets and liabilities: | |||||||||||
| Accounts receivable from Bayer Schering Pharma AG | 8,043,771 | (987,131 | ) | (7,056,640 | ) | ||||||
| Interest receivable | (376,710 | ) | (67,657 | ) | 113,972 | ||||||
| Other current assets | (131,874 | ) | (115,026 | ) | 3,067 | ||||||
| Long term receivable from Bayer Schering Pharma AG | | 87,500 | | ||||||||
| Other long term assets | 20,895 | (356,978 | ) | (139,739 | ) | ||||||
| Accounts payable | 563,958 | (362,027 | ) | 445,310 | |||||||
| Accounts payable to Bayer Schering Pharma AG | (1,473,050 | ) | 1,473,050 | | |||||||
| Accrued expense | (7,786,851 | ) | 7,520,280 | 2,046,338 | |||||||
| Deferred rent | 68,095 | | | ||||||||
| Deferred revenue from Bayer Schering Pharma AG | (11,086,613 | ) | (5,545,919 | ) | 16,632,532 | ||||||
| Other current liabilities | (50,029 | ) | 50,029 | | |||||||
| Other liabilities | | (307,060 | ) | 110,968 | |||||||
| Net cash used in operating activities | (23,335,332 | ) | (19,680,553 | ) | (8,359,213 | ) | |||||
|
Investing activities: |
|||||||||||
| Purchases of capital equipment and leasehold improvements | (1,396,057 | ) | (789,386 | ) | (119,443 | ) | |||||
| Proceeds from sale of capital equipment | | | 4,160 | ||||||||
| Purchases of marketable securities | (46,444,749 | ) | (22,585,008 | ) | | ||||||
| Proceeds from sales of marketable securities | 40,745,514 | | 7,360,968 | ||||||||
| Proceeds from maturities of marketable securities | 946,110 | 372,402 | 12,851,484 | ||||||||
| Net cash (used in) provided by investing activities | (6,149,182 | ) | (23,001,992 | ) | 20,097,169 | ||||||
|
Financing activities: |
|||||||||||
| Proceeds from issuance of common stock under employee benefit plans | 205,325 | 292,113 | 185,117 | ||||||||
| Proceeds from exercise of common stock warrants | 57,440 | 301,330 | 2,351,750 | ||||||||
| Payments on lease obligations | (14,763 | ) | (27,410 | ) | (78,444 | ) | |||||
| Proceeds from issuance of common stock and common stock warrants under equity financings, net of issuance costs | | 28,570,451 | 34,704,619 | ||||||||
| Net cash provided by financing activities | 248,002 | 29,136,484 | 37,163,042 | ||||||||
| Change in cash and cash equivalents for the year | (29,236,512 | ) | (13,546,061 | ) | 48,900,998 | ||||||
| Cash and cash equivalents at beginning of year | 35,771,784 | 49,317,845 | 416,847 | ||||||||
Cash and cash equivalents at end of year |
$ |
6,535,272 |
$ |
35,771,784 |
$ |
49,317,845 |
|||||
Supplemental cash flow information: |
|||||||||||
| Interest paid | $ | 434 | $ | 2,984 | $ | 6,824 | |||||
| Non-cash leasehold incentives provided by landlord | 7,673,040 | | | ||||||||
See accompanying notes.
35
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements
1. Description of Business and Summary of Accounting Policies
Overview
Sonus Pharmaceuticals, Inc. ("Sonus" or the "Company") is developing novel small molecule drugs for the treatment of patients with cancer. Our objective is to identify opportunities where there is the possibility for major improvements in patient treatments and where we believe we can mitigate development risk. We currently have one drug in clinical development and two earlier stage programs. Our plan is to continue to develop our internal pipeline of clinical compound opportunities and to evaluate possible strategic alternatives, including in-licensing, out-licensing and merger and acquisition opportunities, as a means of achieving our business strategies and enhancing stockholder value.
Liquidity
The Company has historically experienced recurring losses from operations which have generated an accumulated deficit of $124.8 million through December 31, 2007. For the year ended December 31, 2007, the Company used $23.3 million of cash to fund operations. At December 31, 2007, the Company had cash, cash equivalents and marketable securities of $34.2 million, and working capital of $28.9 million.
We expect that our cash requirements will decrease in 2008 due to the termination of the development of TOCOSOL Paclitaxel and related staff reductions. Under our current forecasted cash needs, which assume continued development of our lead compound, SN2310, and other earlier stage product candidates, we believe that existing cash, cash equivalents and marketable securities will be sufficient to fund expected operations into the third quarter of 2009. We will need additional capital in 2009 to support the continued development of SN2310, other product candidates and to fund continuing operations.
On November 1, 2007, the Company implemented a reduction of workforce ("Reduction of Workforce") pursuant to which the Company's workforce was reduced by 16 positions, or approximately 25%. The Company undertook the Reduction of Workforce in light of the outcome of its Phase 3 Pivotal Trial for TOCOSOL Paclitaxel. These steps were taken in order to conserve cash and preserve the critical capabilities necessary to pursue the highest priority development programs. Additional information is provided in Note 6.
Cash and Cash Equivalents
Cash and cash equivalents consist of highly liquid investments with a maturity of three months or less at the date of purchase. The Company was invested in $5.3 million in money market funds and $1.0 million in commercial paper at December 31, 2007. The Company had $35.6 million of repurchase agreements as of December 31, 2006.
Fair Value of Financial Instruments
The carrying amounts of certain of the Company's financial instruments, principally cash and cash equivalents, and marketable securities approximate fair value due to their short maturities.
Marketable Securities
The Company classifies the marketable securities portfolio as available-for-sale, and such securities are stated at fair value based on quoted market prices, with the unrealized gains and losses included as
36
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
1. Description of Business and Summary of Accounting Policies (Continued)
a component of accumulated other comprehensive income(loss). Interest earned on securities available-for-sale is included in interest income. The carrying value of debt securities in this category is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion are included in interest income. Realized gains and losses and declines in value judged to be other than temporary on securities available-for-sale also are included in interest income. The cost of securities sold is based on the specific identification method.
Concentrations of Credit Risk
The Company invests its excess cash in accordance with investment guidelines, which limit the credit exposure to any one financial institution other than securities issued by the U.S. government. The guidelines also specify that the financial instruments are issued by institutions with strong credit ratings. These securities generally mature within one year or less and in some cases are not collateralized. At December 31, 2007 the average days to maturity of the Company's portfolio of cash equivalents and marketable securities was 73 days.
Revenue Recognition
Since inception, we have generated revenue from collaborative agreements, licensing fees and from the assignment of developed and patented technology. These arrangements may include upfront non-refundable payments, development milestone payments, payments for research and development services performed and product sales royalties or revenue. Our revenue recognition policies are based on the requirements of SEC Staff Accounting Bulletin No. 104 "Revenue Recognition," and, for contracts with multiple deliverables, we allocate arrangement consideration based on the fair value of the elements under guidance from Emerging Issues Task Force Issue 00-21 ("EITF 00-21"), "Revenue Arrangements with Multiple Deliverables." Under EITF 00-21, revenue arrangements with multiple deliverables are divided into separate units of accounting and revenue is allocated to these units based upon relative fair values with revenue recognition criteria considered separately for each unit.
Nonrefundable upfront technology license fees, for product candidates where we are providing continuing services related to product development, are deferred and recognized as revenue over the estimated development period.
The timing and amount of revenue that we recognize from licenses of technology, either from upfront fees or milestones where we are providing continuing services related to product development, is primarily dependent upon our estimates of the development period. We define the development period as the point from which research activities commence up to FDA approval of our submission assuming no further research is necessary. As product candidates move through the development process, it is necessary to revise these estimates to consider changes to the product development cycle, such as changes in the clinical development plan, regulatory requirements, or various other factors, many of which may be outside of our control. Should our clinical development plans change, as a result of regulatory or other matters, we would adjust our development period estimates accordingly. The impact on revenue of changes in our estimates and the timing thereof is recognized prospectively over the remaining estimated product development period. Revenue from research and development services performed under collaboration agreements is generally recognized in the period when the services are performed. Payments received in excess of amounts earned are recorded as deferred revenue.
37
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
1. Description of Business and Summary of Accounting Policies (Continued)
Research and Development Costs
Research and development ("R&D") costs including personnel costs, supplies, depreciation and other indirect costs are expensed as incurred. Costs are expensed the earlier of when amounts are due or when services are performed. R&D expenses consist of independent R&D costs and costs associated with collaborative R&D arrangements.
Other (expense) income
Other (expense) income includes net transaction gains and losses on foreign denominated payables of approximately ($125,000), ($115,000) and $0 in 2007, 2006 and 2005, respectively.
Equipment, Furniture and Leasehold Improvements
Equipment, furniture and leasehold improvements are stated at cost. Depreciation is provided using the straight-line basis generally over three years for equipment and 5 years for furniture and fixtures which represents the estimated useful life of the assets. Leasehold improvements are amortized over the lesser of the economic useful lives of the improvements or the term of the related lease. The current lease has 10 years remaining. Repair and maintenance costs are expensed as incurred.
Segment Information
The Company follows the requirements of SFAS No. 131, "Disclosure About Segments of an Enterprise and Related Information." The Company has one operating segment, the development of oncology drugs.
Stock-Based Compensation
The Company adopted the requirements of SFAS No. 123 (revised 2004), "Share-Based Payment," (or "SFAS 123R") on January 1, 2006, utilizing the "modified prospective" method. The Company uses the Black-Scholes-Merton option pricing model as the most appropriate fair-value method for its awards and recognizes compensation cost on a straight-line basis over its awards' vesting periods in accordance with the provisions of SFAS 123R. In valuing its options using the Black-Scholes-Merton option pricing model, the Company makes assumptions about risk-free interest rates, dividend yields, volatility and weighted average expected lives, including estimated forfeiture rates, of the options. Risk-free interest rates are derived from United States treasury securities as of the option grant date. Dividend yields are based on the Company's historical dividend payments, which have been zero to date. Volatility is derived from the historical volatility of the Company's common stock as traded on NASDAQ. Forfeiture rates are estimated using historical actual forfeiture rates that resulted over the estimated life of the option grant for options granted as of the beginning of the forfeiture measurement period. These rates are adjusted on a quarterly basis and any change in compensation expense is recognized in the period of the change. The weighted average expected life of the options is based on historical experience of option exercises and the average vesting option schedule. In November 2005, the FASB issued FASB Staff Position No. 123R-3, "Transition Election Related to Accounting for the Tax Effects of Share-Based Payment Awards". The Company has adopted the simplified method to calculate the beginning balance of the additional paid-in-capital (or "APIC") pool of excess tax benefit, and to determine the subsequent effect on the APIC pool and the Statements of Cash Flows of the tax effects of stock-based compensation awards that were outstanding upon our adoption of SFAS 123R.
38
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
1. Description of Business and Summary of Accounting Policies (Continued)
For unvested awards granted prior to the adoption date, compensation expense is based on the original grant date fair value measurement under SFAS 123, "Accounting for Stock-Based Compensation".
Income Taxes
The Company utilizes the liability method of accounting for income taxes as required by SFAS No. 109, "Accounting for Income Taxes." Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Due to uncertainty of the Company's ability to generate taxable income, a full valuation allowance has been established as of December 31, 2007.
Effective January 1, 2007, the Company adopted the provisions of Financial Interpretation No. 48, "Accounting for Uncertainty in Income Taxesan interpretation of FASB Statement No. 109" ("FIN 48"). FIN 48 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. At the date of adoption of FIN 48, we had no unrecognized tax benefits and expected no significant changes in unrecognized tax benefits in the next twelve months. The adoption of this statement did not result in a cumulative accounting adjustment and did not impact our financial position, results of operations or cash flows.
Comprehensive Income
In accordance with Statement of Financial Accounting Standard No. 130, "Reporting Comprehensive Income" (SFAS 130), the Company has reported comprehensive income, defined as net income (loss) plus other comprehensive income(loss), in the Statements of Stockholders' Equity. The total of other accumulated comprehensive income(loss) consists of unrealized gains and losses on certain cash equivalents and marketable securities.
Per Share Data
Basic net loss per share is based on the weighted average number of common shares outstanding. Diluted net loss per share is based on the weighted average number of common shares and dilutive potential common shares. Dilutive potential common shares are calculated under the treasury stock method and consist of unexercised stock options and warrants.
Use of Estimates and Reclassifications
The preparation of financial statement in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Certain reclassifications of prior period amounts have been made to our financial statements to conform to the current period presentation.
39
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
1. Description of Business and Summary of Accounting Policies (Continued)
Recent Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board ("FASB") issued SFAS No. 157, "Fair Value Measurements," which establishes a framework for measuring fair value, and expands disclosures about fair value measurements. This statement does not require any new fair value measurements, but increases consistency and comparability in the use of fair value measurements and calculations. This statement is effective for financial statements issued for fiscal years beginning after November 15, 2007 and for interim periods within those fiscal years. Management does not anticipate that the adoption of SFAS No. 157 will have a material effect on our financial position or results of operations.
In February 2007, the FASB issued SFAS No. 159, "The Fair Value Option for Financial Assets and Financial LiabilitiesIncluding an amendment to FASB Statement No. 115". SFAS 159 allows companies to choose to measure eligible assets and liabilities at fair value with changes in value recognized in earnings. Fair value treatment for eligible assets and liabilities may be elected either prospectively upon initial recognition, or if an event triggers a new basis of accounting for an existing asset or liability. SFAS 159 is effective in the first quarter of 2008, and the Company is currently evaluating the impact of adoption on its financial position and results of operations.
In June 2007, the EITF reached a consensus on EITF No. 07-03, Accounting for Nonrefundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities, or EITF 07-03. EITF 07-03 requires that nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities be deferred and capitalized and recognized as an expense as the goods are delivered or the related services are performed. EITF 07-03 is effective for fiscal years beginning after December 15, 2007, and will be adopted by the Company in the first quarter of 2008. The adoption of EITF 07-3 will have the effect of changing our policy on nonrefundable prepayments for research and development services whereby such costs will be deferred and recognized as the services are rendered as compared to the existing policy whereby such payments are charged to research and development expense as paid. This change may have an impact on financial condition and the results of operations in future periods.
In December 2007, the EITF reached a consensus on EITF No. 07-01, Accounting for Collaborative Arrangements Related to the Development and Commercialization of Intellectual Property, or EITF 07-01. EITF 07-01 discusses the appropriate income statement presentation and classification for the activities and payments between the participants in arrangements related to the development and commercialization of intellectual property. The sufficiency of disclosure related to these arrangements is also specified. EITF 07-01 is effective for fiscal years beginning after December 15, 2008. As a result, EITF 07-01 is effective for us in the first quarter of fiscal 2009. We do not expect the adoption of EITF 07-01 to have a material impact on either our financial position or results of operations.
2. Collaboration and License Agreement with Bayer Schering
On October 17, 2005, the Company entered into a Collaboration and License Agreement with Bayer Schering, a German corporation, pursuant to which, among other things, the Company granted Bayer Schering an exclusive, worldwide license to TOCOSOL Paclitaxel, its anti-cancer product candidate (the "Product"). With respect to the Product, Bayer Schering paid Sonus an upfront license fee of $20 million and paid Sonus for research and development services performed equal to 50% of eligible product research and development costs (in certain cases the reimbursement rate was 100%).
40
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
2. Collaboration and License Agreement with Bayer Schering (Continued)
In connection with the Collaboration and Licensing Agreement, the Company and an affiliate of Bayer Schering entered into a Securities Purchase Agreement whereby the Company sold 3,900,000 shares of common stock for an aggregate of $15.7 million and warrants to purchase 975,000 shares of common stock for an aggregate purchase price of $122,000.
On October 3, 2007, Sonus received notification from Bayer Schering of its decision to terminate the Collaboration and License Agreement in accordance with its terms because the Phase 3 pivotal trial did not meet its primary endpoint and the results of the trial do not support, in Bayer Schering's judgment, a submission for a New Drug Application with the United States Food and Drug Administration ("FDA"). The termination was effective on November 2, 2007. In accordance with the terms of the Agreement, all rights to TOCOSOL Paclitaxel have reverted back to Sonus. The Company has discontinued development of TOCOSOL Paclitaxel due to results of the Phase 3 study. These closure activities were substantially complete by December 31, 2007.
Due to the termination of the Agreement, in the fourth quarter of 2007 the Company recognized as revenue the balance of the unamortized deferred revenue from the upfront license fee. Revenue recognized from the amortization of the deferred revenue from the upfront license fee was $11.0 million, $5.5 million and $1.2 million in 2007, 2006 and 2005, respectively. The Company reduced the revenue to be recognized over the development period related to the $20 million upfront license payment by $2.3 million, which represented the excess fair value of the warrants purchased by an affiliate of Bayer Schering above the amount paid in connection with its equity investment in Sonus. This adjustment was made because both the equity investment and the upfront payment were considered to be a single unit of accounting.
The Company recognized revenue of $9.1 million, $16.9 million and $7.1 million in 2007, 2006 and 2005, respectively for reimbursement of expenses related to research and development services performed for the Phase 3 trial for TOCOSOL Paclitaxel and related drug supply and manufacturing costs, including expenses associated with the termination of the Phase 3 trial. In addition, the Company recognized expenses of $4.1 million in 2007 and $1.7 million in 2006 for the Company's share of development expenses incurred by Bayer Schering in accordance with the terms of the Agreement. There were no such expenses in 2005.
The Company does not expect to earn revenue or incur expense related to the Agreement with Bayer Schering beyond 2007. The final net billing between the Company and Bayer Schering was completed during the fourth quarter of 2007. Settlement of the final amount outstanding was received from Bayer Schering in December 2007. No receivables from, or payables to, Bayer Schering are outstanding at December 31, 2007.
41
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
3. Marketable Securities
Marketable securities consist of the following:
|
|
Cost |
Unrealized Gains |
Unrealized Losses |
Fair Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007: | ||||||||||||
| Corporate debt securities | $ | 26,019,825 | $ | 1,910 | | $ | 26,021,735 | |||||
| Government debt securities | 1,344,037 | 160 | | 1,344,197 | ||||||||
| Asset-backed securities | 298,342 | | (720 | ) | 297,622 | |||||||
| $ | 27,662,204 | $ | 2,070 | $ | (720 | ) | $ | 27,663,554 | ||||
|
2006: |
||||||||||||
| Corporate debt securities | $ | 21,100,297 | $ | 3,068 | $ | (1,111 | ) | $ | 21,102,254 | |||
| Asset-backed securities | 1,406,481 | | (2,649 | ) | 1,403,832 | |||||||
| $ | 22,506,778 | $ | 3,068 | $ | (3,760 | ) | $ | 22,506,086 | ||||
There were no significant realized or unrealized gains or losses on the sales of marketable securities in 2007, 2006 or 2005. All of the marketable securities held as of December 31, 2007 had maturities of one year or less. The Company only invests in A (or equivalent) rated securities with maturities of one year or less. The Company does not believe that there are any permanent impairments related to unrealized losses for the year ended December 31, 2007 given the quality of the investment portfolio and its short-term nature.
4. Equipment, Furniture and Leasehold Improvements
Equipment, furniture and leasehold improvements consist of the following:
|
|
2007 |
2006 |
|||||
|---|---|---|---|---|---|---|---|
| Laboratory equipment | $ | 3,378,189 | $ | 3,973,654 | |||
| Office furniture and equipment | 885,455 | 1,584,861 | |||||
| Leasehold improvements | 7,673,040 | 1,390,879 | |||||
| 11,936,684 | 6,949,394 | ||||||
| Less accumulated depreciation and amortization | (3,293,885 | ) | (5,763,220 | ) | |||
| 8,642,799 | 1,186,174 | ||||||
| Construction in progress | 934,768 | | |||||
| $ | 9,577,567 | $ | 1,186,174 | ||||
We held laboratory equipment acquired under capital leases with an original cost of $392,968 as of December 31, 2007 and 2006. Accumulated depreciation on this equipment was $392,968 and $380,500 at December 31, 2007 and 2006, respectively.
During 2007, in preparation for a move to a new facility, the Company disposed of $1,756,163 of furniture, fixtures and equipment that would no longer be utilized by the Company. Accumulated depreciation on this equipment was $1,737,954 at the time of disposal. In addition, at the expiration of its facilities lease the Company abandoned leasehold improvments that had been made to the facility of
42
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
4. Equipment, Furniture and Leasehold Improvements (Continued)
$1,390,879. Accumulated amortization of these leasehold improvements at the lease expiration was $1,387,073.
5. Accrued Expenses
Accrued expenses consist of the following:
|
|
2007 |
2006 |
||||
|---|---|---|---|---|---|---|
| Clinical trials | $ | 2,627,765 | $ | 8,497,278 | ||
| Product manufacturing | | 1,617,580 | ||||
| Severance | 908,496 | | ||||
| Compensation | 227,044 | 1,459,128 | ||||
| Other | 377,968 | 354,138 | ||||
| $ | 4,141,273 | $ | 11,928,124 | |||
6. Reduction of Workforce
On November 1, 2007, the Company implemented a reduction of workforce ("Reduction of Workforce") pursuant to which the Company's workforce was reduced by 16 positions, or approximately 25%. The effective date of the Reduction of Workforce was November 30, 2007. The Company announced the Reduction of Workforce in light of the outcome of its Phase 3 Pivotal Trial for TOCOSOL Paclitaxel. These steps were taken in order to conserve cash and preserve the critical capabilities necessary to pursue the highest priority development programs. The total cost of the Reduction of Workforce was approximately $1.2 million, which consisted of payments for severance and medical insurance and was recognized as expense in the fourth quarter of 2007. Severance expense of approximately $575,000 and $625,000 was recognized in research and development expense and general and administrative expense, respectively, in the fourth quarter of 2007. The following table summarizes the severance expense activity:
| Severance expense recorded in 2007 | $ | 1,192,659 | ||
| Cash Payments made in 2007 | 284,163 | |||
| Accrued severance as of December 31, 2007 | $ | 908,496 | ||
The severance accrual as of December 31, 2007 was paid in the first quarter of 2008.
7. Other assets
Other assets consist of the following:
|
|
2007 |
2006 |
||||
|---|---|---|---|---|---|---|
| Deposit on facility lease | $ | 439,822 | $ | 439,822 | ||
| Long-term portion of prepaid insurance | | 20,895 | ||||
| $ | 439,822 | $ | 460,717 | |||
43
Notes to Financial Statements (Continued)
8. Income Tax
The Company recorded no income tax expense or benefit during 2007, 2006 or 2005.
A reconciliation of the Federal Statutory tax rate of 34% to the Company's effective income tax rate follows:
|
|
2007 |
2006 |
2005 |
||||
|---|---|---|---|---|---|---|---|
| Statutory tax rate | (34.00 | )% | (34.00 | )% | (34.00 | )% | |
| Research Credits | 12.61 | (1.99 | ) | (1.67 | ) | ||
| Permanent difference | 0.05 | 0.04 | 3.67 | ||||
| Change in valuation allowance | 19.87 | 35.08 | 33.63 | ||||
| Other | 1.47 | .87 | (1.63 | ) | |||
| Effective tax rate | | | | ||||
Significant components of the Company's net deferred tax assets and liabilities as of December 31, 2007 and 2006 are as follows:
|
|
2007 |
2006 |
|||||
|---|---|---|---|---|---|---|---|
| Deferred tax assets: | |||||||
| Federal net operating loss carryforwards | $ | 40,251,000 | $ | 32,863,000 | |||
| Deferred Revenue | | 3,769,000 | |||||
| Accrued expenses | 239,000 | 195,000 | |||||
| Research and development credits | 1,471,000 | 3,119,000 | |||||
| Stock Options | 1,304,000 | 739,000 | |||||
| Book depreciation expense in excess of tax depreciation expense | (48,000 | ) | (62,000 | ) | |||
| Gross deferred tax assets | 43,217,000 | 40,623,000 | |||||
| Valuation allowance for net deferred tax assets | (43,217,000 | ) | (40,623,000 | ) | |||
| Net deferred tax assets | $ | | $ | | |||
Due to the uncertainty of the Company's ability to generate taxable income to realize its net deferred tax assets at December 31, 2007 and 2006, a valuation allowance has been recognized for financial reporting purposes. The Company's valuation allowance for deferred tax assets increased $2.6 million and $8.3 million for the years ended December 31, 2007 and 2006, respectively. The increase in the deferred tax assets in 2007 is primarily the result of increasing net operating loss carryforwards.
At December 31, 2007 the Company has federal net operating loss carryforwards of approximately $119 million for income tax reporting purposes and research and development tax credit carryforwards of approximately $1.5 million. The federal operating loss carryforwards and research and development credits will expire between 2008 and 2028. To the extent that net operating loss carryforwards, when realized, relate to stock option deductions of approximately $3 million, the resulting benefit will be credited to stockholders' equity.
The initial public offering of common stock by the Company in 1995 caused an ownership change pursuant to applicable regulations in effect under the Internal Revenue Code of 1986. Therefore, the
44
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
8. Income Tax (Continued)
Company's use of losses incurred through the date of ownership change will be limited during the carryforward period and may result in the expiration of net operating loss carryforwards before utilization.
The Company adopted the provisions of FASB Interpretation No. 48 Accounting for Uncertainty in Income Taxes, on January 1, 2007. The Company has no unrecognized tax benefits which would require an adjustment to the January 1, 2007 beginning balance of retained earnings. The Company had no unrecognized tax benefits at January 1, 2007 and at December 31, 2007.
The Company recognizes interest accrued and penalties related to unrecognized tax benefits in tax expense. During the years ended December 31, 2007 and 2006, the Company recognized no interest and penalties.
9. Stockholders' Equity
Common Stock
At December 31, 2007, the Company had shares of common stock reserved for possible future issuance as follows:
| Stock options outstanding | 4,278,960 | |
| Warrants outstanding | 4,080,533 | |
| Shares available for future grant under stock plans | 5,325,366 | |
| 13,684,859 | ||
Common Stock Issuances
In May 2006, the Company issued approximately 6.1 million shares of common stock in a registered direct offering for gross proceeds of $30.6 million (approximately $28.6 million net of transaction costs). The common stock was sold at a price of $5.00 per share and was previously registered through a shelf registration statement on Form S-3 that was declared effective by the SEC in April 2006.
In October 2005, the Company issued 3,900,000 shares of common stock and warrants to purchase 975,000 shares of common stock to Schering Berlin Venture Corporation for aggregate consideration of $15.8 million in connection with the Collaboration and License Agreement with Bayer Schering. The common stock was sold at $4.02 per share, which was equal to the per share closing price of the Company's common stock as reported on the Nasdaq National Market on October 14, 2005, the trading day immediately preceding the date of the Securities Purchase Agreement. The five-year warrants were sold at a price of $0.125 per share underlying each warrant, have an exercise price of $4.42 per share and expire in October 2010.
In August 2005, the Company sold 4.7 million shares of common stock and warrants to purchase up to 2.3 million shares of common stock in a private placement transaction for gross proceeds of $17.8 million (approximately $16.6 million net of transaction costs). The common stock was sold at a price of $3.77 per share. The five-year warrants were sold at a price of $0.125 per share underlying each warrant, have an exercise price of $4.15 per share and expire in August 2010.
45
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
9. Stockholders' Equity (Continued)
Stock Warrants
At December 31, 2007, there were warrants outstanding to purchase 4.1 million shares of common stock at exercise prices ranging from $4.09 to $4.42 per share and expiration dates ranging from July 2008 to October 2010. During 2007, the Company recorded $57,440 in proceeds from the issuance of 14,044 shares of common stock from the exercise of common stock warrants. During 2006, the Company recorded $301,330 in proceeds from the issuance of 73,675 shares of common stock from the exercise of common stock warrants.
Stock Options
The Company has stock option plans whereby shares of common stock are reserved for future issuance pursuant to stock option grants or other issuances. Under the 2000 Stock Incentive Plan, an incremental number of shares equal to four percent of the Company's common stock outstanding as of December 31 of each year commencing December 31, 2000 are made available for issuance under the plan up to a lifetime maximum of five million shares. The Company reached the lifetime cap in 2006. In 2007 Sonus shareholders approved a new incentive plan entitled the "2007 Performance Incentive Plan." Under the term of this plan the Company can issue up to 3,900,000 additional shares of the Company's common stock through the grant of stock options and restricted stock. Employee stock options vest over a period of time determined by the Board of Directors, generally four years, and director stock options are generally fully vested on the date of grant. Stock options generally are granted at the fair market value on the date of grant and expire ten years from the date of grant.
Adoption of SFAS 123R
The Company adopted the requirements of SFAS No. 123 (revised 2004), "Share-Based Payment," (or "SFAS 123R") on January 1, 2006, utilizing the "modified prospective" method. The Company uses the Black-Scholes-Merton option pricing model as the most appropriate fair-value method for its awards and recognizes compensation cost on a straight-line basis over its awards' vesting periods in accordance with the provisions of SFAS 123R. In valuing its options using the Black-Scholes-Merton option pricing model, the Company makes assumptions about risk-free interest rates, dividend yields, volatility and weighted average expected lives, including estimated forfeiture rates, of the options. Risk-free interest rates are derived from United States treasury securities as of the option grant date. Dividend yields are based on the Company's historical dividend payments, which have been zero to date. Volatility is derived from the historical volatility of the Company's common stock as traded on NASDAQ. Forfeiture rates are estimated using historical actual forfeiture rates that resulted over the estimated life of the option grant for options granted as of the beginning of the forfeiture measurement period. These rates are adjusted on a quarterly basis and any change in compensation expense is recognized in the period of the change. The weighted average expected life of the options is based on historical experience of option exercises and the average vesting option schedule.
For unvested awards granted prior to the adoption date, compensation expense is based on the original grant date fair value measurement under SFAS 123, "Accounting for Stock-Based Compensation". We currently believe that the assumptions used to generate those fair values are appropriate and therefore have not revised those calculations.
46
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
9. Stockholders' Equity (Continued)
Prior to the adoption of SFAS 123R
The Company previously applied Accounting Principles Board (APB) Opinion No. 25, "Accounting for Stock Issued to Employees," and related Interpretations and provided the required pro forma disclosures of SFAS No. 123.
The pro forma information for the year ended December 31, 2005 was as follows:
|
|
2005 |
|||
|---|---|---|---|---|
| Net loss, as reported | $ | (21,097,017 | ) | |
| Add: Stock-based employee compensation expense included in reported net loss | | |||
Deduct: Stock-based employee compensation expense determined under fair value based method |
(1,629,317 |
) |
||
Pro forma net loss |
$ |
(22,726,334 |
) |
|
Loss per share: |
||||
| Basic and diluted-as reported | $ | (0.88 | ) | |
| Basic and diluted-pro forma | $ | (0.95 | ) | |
Impact of the adoption of SFAS 123R
The Company elected to implement SFAS 123R using the modified prospective application method. Accordingly, during the year ended December 31, 2006, the Company recorded stock-based compensation expense totaling the amount that would have been recognized had the fair value method been applied since the effective date of SFAS 123 for unvested options outstanding as of January 1, 2006 and recorded compensation expense under the provisions of SFAS 123R for options granted during the years ended December 31, 2007 and 2006. Previously reported amounts have not been restated. As the Company uses a full valuation allowance with respect to deferred taxes, the adoption of SFAS 123R had no impact on deferred taxes or cash flow.
The effect of recording stock-based compensation for the periods ended December 31, 2007 and December 31, 2006 was as follows:
|
|
2007 |
2006 |
||||||
|---|---|---|---|---|---|---|---|---|
| Stock-based compensation expense: | ||||||||
| General & administrative | $ | (1,080,455 | ) | $ | (1,105,253 | ) | ||
| Research & development | (580,740 | ) | (1,068,126 | ) | ||||
| Total stock-based compensation expense | (1,661,195 | ) | (2,173,379 | ) | ||||
| Tax effect on stock-based compensation | | | ||||||
| Net effect on income | $ | (1,661,195 | ) | $ | (2,173,379 | ) | ||
Effect on earnings per share: |
||||||||
| Basic and diluted | $ | (0.05 | ) | $ | (0.06 | ) | ||
47
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
9. Stockholders' Equity (Continued)
As of January 1, 2006, the Company had an unrecorded deferred stock-based compensation balance related to stock options of $5.4 million before estimated forfeitures. In the Company's pro forma disclosures prior to the adoption of SFAS 123R, the Company accounted for forfeitures upon occurrence. SFAS 123R requires forfeitures to be estimated at the time of grant and revised if necessary in subsequent periods if actual forfeitures differ from those estimates. Accordingly, as of January 1, 2006, the Company estimated that the stock-based compensation for the awards not expected to vest was $1.2 million, and therefore, the pro forma deferred stock-based compensation balance related to stock options was adjusted to $4.2 million after estimated forfeitures.
As of December 31, 2007, the pro forma deferred stock-based compensation balance related to stock options after adjusting for estimated forfeitures was $3.5 million and will be recognized over an estimated weighted average period of 2.0 years.
The reduction of expense in the research & development area in 2007 related to the impact of a mark to market adjustment for consultant option awards. These awards are revalued at the end of each quarter. The significant decline in the Company's stock price in 2007 resulted in a decrease of approximately $200,000 in stock compensation expense as compared to 2006.
The fair value of each stock option used in the calculations under SFAS 123R is estimated using the Black-Scholes-Merton option pricing model. The assumptions used in this model include (1) the stock price at grant date, (2) the exercise price, (3) an estimated option life of four to 6.59 years, four years and four years in 2007, 2006 and 2005, respectively, (4) no expected dividends for each period presented, (5) stock price volatility factor of 1.01%, 62.9% and 78.7% in 2007, 2006 and 2005, respectively, and (6) a risk-free interest rate of 4.0%, 4.6% and 4.4% in 2007, 2006 and 2005, respectively.
The Company's change in the estimated forfeiture rate in 2007 was based on personnel reductions in the fourth quarter of 2007, and resulted in a decrease of approximately $445,000 in stock compensation expense as compared to 2006. This change in estimate was based on events which occurred or were triggered in the third quarter 2007.
The Black-Scholes-Merton option pricing model was developed for use in estimating the fair value of short-lived exchange traded options that have no vesting restrictions and are fully transferable. In addition, option pricing models require the input of highly subjective assumptions, including the option's expected life and the price volatility of the underlying stock. The Company will evaluate its assumptions on a regular basis. These evaluations may result in changes to assumptions which may have a material effect on compensation expense recorded under SFAS 123R.
48
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
9. Stockholders' Equity (Continued)
A summary of activity related to the Company's stock options follows:
|
|
Shares |
Exercise Price |
|||
|---|---|---|---|---|---|
| Balance, December 31, 2004 | 3,010,509 | 0.63 - 44.00 | |||
| Granted | 1,039,000 | 2.87 - 5.10 | |||
| Exercised | (60,998 | ) | 0.88 - 4.06 | ||
| Canceled | (169,341 | ) | 2.03 - 8.19 | ||
| Balance, December 31, 2005 | 3,819,170 | 0.63 - 44.00 | |||
| Granted | 1,023,650 | 4.48 - 6.11 | |||
| Exercised | (53,720 | ) | 0.88 - 3.86 | ||
| Canceled | (32,210 | ) | 2.30 - 20.50 | ||
| Balance, December 31, 2006 | 4,756,890 | 0.63 - 44.00 | |||
| Granted | 101,750 | 5.03 - 5.74 | |||
| Exercised | (35,937 | ) | 5.36 - 5.78 | ||
| Canceled | (543,743 | ) | 2.30 - 44.00 | ||
| Balance, December 31, 2007 | 4,278,960 | .63 - 19.38 | |||
Options exercisable at December 31, 2007, 2006, and 2005, were 3,356,015, 2,618,765 and 1,953,680, respectively. The weighted average exercise prices for those options for the years ended December 31, 2007, 2006 and 2005, were $4.69, $4.72 and $4.83, respectively.
The intrinsic value of options exercised during 2007, 2006 and 2005 was $154,353, $178,220 and $109,644, respectively. The estimated fair value of shares vested during 2007, 2006 and 2005 was $3,099,433, $2,310,842 and $2,186,496, respectively. The weighted-average estimated fair value of stock options granted during 2007, 2006 and 2005 was $2.57, $3.09 and $2.99, respectively, based on the assumptions in the Black-Scholes-Merton valuation model discussed above.
49
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
9. Stockholders' Equity (Continued)
The following table summarizes information about stock options outstanding at December 31, 2007:
|
|
Options Outstanding |
|
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
Weighted- Average Remaining Contractual Life (in years) |
|
|
|
|
||||||||
|
|
|
|
|
Options Exercisable |
||||||||||
|
Range of Exercise Prices |
Number of Shares Outstanding |
Weighted- Average Exercise Price |
Number of Shares Outstanding |
Weighted- Average Remaining Contractual Life (in years) |
Weighted- Average Exercise Price |
|||||||||
| $0.63 - $2.30 | 557,524 | 4.25 | $ | 1.70 | 557,524 | 4.25 | $ | 1.70 | ||||||
| $2.30 - $3.23 | 520,560 | 6.96 | $ | 3.10 | 429,660 | 6.97 | $ | 3.10 | ||||||
| $3.23 - $5.01 | 728,720 | 6.00 | $ | 4.54 | 695,003 | 5.91 | $ | 4.54 | ||||||
| $5.01 - $5.08 | 21,000 | 7.91 | $ | 5.05 | 8,500 | 5.95 | $ | 5.08 | ||||||
| $5.08 - $5.10 | 720,726 | 7.96 | $ | 5.10 | 425,476 | 7.96 | $ | 5.10 | ||||||
| $5.10 - $6.00 | 592,495 | 5.97 | $ | 5.66 | 480,681 | 5.38 | $ | 5.73 | ||||||
| $6.00 - $6.11 | 501,961 | 8.99 | $ | 6.11 | 125,489 | 9.00 | $ | 6.11 | ||||||
| $6.11 - $6.75 | 428,133 | 3.10 | $ | 6.59 | 426,258 | 3.08 | $ | 6.59 | ||||||
| $6.75 - $8.08 | 197,841 | 3.88 | $ | 7.86 | 197,424 | 3.87 | $ | 7.86 | ||||||
| $19.38 - $19.38 | 10,000 | .33 | $ | 19.38 | 10,000 | .33 | $ | 19.38 | ||||||
| 4,278,960 | 6.17 | $ | 4.82 | 3,356,015 | $ | 4.69 | ||||||||
At December 31, 2007, the aggregate intrinsic value of the outstanding options was $0 and the aggregate intrinsic value of the exercisable options was $0.
Stock Purchase Plan
The Company has an employee stock purchase plan whereby employees may contribute up to 15% of their compensation to purchase shares of the Company's common stock at 85% of the stock's fair market value at the lower of the beginning or end of each six-month offering period. Shares purchased under the plan were 46,807, 13,642 and 6,493 in 2007, 2006 and 2005, respectively. At December 31, 2007, a total of 39,551 shares remain available for purchase by employees under the plan. The previous plan expired on December 31, 2005 and a new plan was approved by the shareholders at the 2006 annual meeting with a ten year term.
401(k) Plan
The Company has a 401(k) plan for all employees under which it provides a specified percentage match on employee contributions. Currently, the Company match is made in shares of the Company's common stock. Shares issued as matching contributions under the plan were 96,573, 17,191 and 18,591 in 2007, 2006 and 2005, respectively. The related expense recorded on these matching contributions was $103,697, $92,762 and $66,767 in 2007, 2006 and 2005, respectively. At December 31, 2007, a total of 14,714 shares remain available for future issuances as matching contributions under the plan.
Shareholder Rights Plan
The Company has adopted a Shareholder Rights Plan ("Plan") which was amended in July 2002 and more recently in August 2006. Under the Plan, as amended, the Company's Board of Directors
50
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
9. Stockholders' Equity (Continued)
declared a dividend of one Preferred Stock Purchase Right ("Right") for each outstanding common share of the Company. The Rights have an exercise price of $140 per Right and provide the holders with the right to purchase, in the event a person or group acquires 15% or more of the Company's common stock, additional shares of the Company's common stock having a market value equal to two times the exercise price of the Right. The Rights expire in 2016.
10. Net Loss Per Share
The following table presents the computation of basic and diluted net loss per share:
|
|
2007 |
2006 |
2005 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basic and diluted net loss per share: | |||||||||||
| Net loss | $ | (13,063,168 | ) | $ | (23,551,296 | ) | $ | (21,097,017 | ) | ||
| Weighted average common shares | 36,909,462 | 34,729,930 | 24,027,127 | ||||||||
| Basic and diluted net loss per share | $ | (0.35 | ) | $ | (0.68 | ) | $ | (0.88 | ) | ||
As of December 31, 2007, 2006 and 2005 a total of 8,359,493, 9,237,267 and 8,373,322 options and warrants, respectively, have not been included in the calculation of potential common shares as their effect on diluted per share amounts would have been anti-dilutive.
11. Commitments and Contingencies
The Company has leased office space under a non-cancelable operating lease expiring in 2017 and office equipment under two non-cancelable operating leases which expire in 2009 and 2010. Rental expense for the years ended December 31, 2007, 2006 and 2005 was $784,000, $655,000 and $644,000, respectively.
In November 2006 the Company entered into a new operating lease agreement for combined laboratory and office space. Our previous operating lease for facilities expired December 31, 2007, and we moved into the newly leased facility in December 2007. The new lease, as amended in 2007, is for approximately 42,600 square feet and expires on December 31, 2017, with a provision for two additional five year renewals. In connection with the new lease, we received landlord-provided incentives of approximately $7.7 million in the form of tenant improvements, which have been recorded as additions to fixed assets and deferred rent liabilities and will be amortized over the term of the lease. In connection with our new lease arrangement, we were required to provide a cash security deposit of approximately $497,000 of which approximately $440,000 was paid upon lease signing in November 2006, and the remainder was paid in February 2008. In addition, the lease stipulates the Company must issue a standby letter of credit for approximately $500,000 which is expected to be issued during the first quarter of 2008.
51
Sonus Pharmaceuticals, Inc.
Notes to Financial Statements (Continued)
11. Commitments and Contingencies (Continued)
Future minimum lease payments under these leases are as follows:
| 2008 | $ | 1,929,120 | |
| 2009 | 1,981,996 | ||
| 2010 | 1,997,220 | ||
| 2011 | 2,055,144 | ||
| 2012 | 2,116,800 | ||
| Thereafter | 11,575,536 | ||
| $ | 21,655,816 | ||
12. Subsequent Event
In March 2007, Bristol-Myers Squibb Pharmaceuticals recalled certain batches of Taxol due to potential lack of sterility assurance. Sonus had some of these batches at clinical sites which were being used in the reference arm of the Phase 3 TOCOSOL Paclitaxel pivotal study. The Company has returned all of the recalled material to its suppliers in accordance with the recall notice. On March 12, 2008, the Company received an initial refund from its suppliers of approximately $850,000 for returned material.
13. Quarterly Financial Information (unaudited)
|
|
Quarter Ended |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Mar. 31 |
June 30 |
Sept. 30 |
Dec. 31 |
|||||||||||
|
|
(in thousands, except per share data) |
||||||||||||||
| 2007 | |||||||||||||||
| Collaboration revenue from Bayer Schering Pharma AG | $ | 5,051 | $ | 3,271 | $ | 4,079 | $ | 7,730 | |||||||
| Operating expenses | $ | 8,915 | $ | 9,825 | $ | 10,433 | $ | 6,193 | |||||||
| Operating income (loss) | $ | (3,864 | ) | $ | (6,554 | ) | $ | (6,354 | ) | $ | 1,537 | ||||
| Net income (loss) | $ | (3,225 | ) | $ | (5,963 | ) | $ | (5,808 | ) | $ | 1,933 | ||||
| Net income (loss) per share*: | |||||||||||||||
| Basic and diluted | $ | (0.09 | ) | $ | (0.16 | ) | $ | (0.16 | ) | $ | .05 | ||||
2006 |
|||||||||||||||
| Collaboration revenue from Bayer Schering Pharma AG | $ | 4,054 | $ | 7,514 | $ | 4,931 | $ | 5,893 | |||||||
| Operating expenses | $ | 9,879 | $ | 13,136 | $ | 12,067 | $ | 13,596 | |||||||
| Operating loss | $ | (5,825 | ) | $ | (5,623 | ) | $ | (7,136 | ) | $ | (7,703 | ) | |||
| Net loss | $ | (5,310 | ) | $ | (4,935 | ) | $ | (6,293 | ) | $ | (7,013 | ) | |||
| Net loss per share*: | |||||||||||||||
| Basic and diluted | $ | (0.17 | ) | $ | (0.14 | ) | $ | (0.17 | ) | $ | (0.19 | ) | |||
- *
- Quarterly EPS may not add to annual figure due to rounding.
52
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934 (the "Exchange Act")), as of the end of the period covered by this annual report on Form 10-K. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of such date, our disclosure controls and procedures were effective.
In addition, no change in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) occurred during the fourth quarter of our fiscal year ended December 31, 2007 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
53
Management's Report on Internal Control Over Financial Reporting
The management of Sonus Pharmaceuticals, Inc. (the "Company") is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. The Company's internal control over financial reporting is a process designed under the supervision of the Company's principal executive and principal financial officers to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the Company's financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles.
As of December 31, 2007, management assessed the effectiveness of the Company's internal control over financial reporting based on the framework established in "Internal ControlIntegrated Framework" issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation, management has determined that the Company's internal control over financial reporting was effective as of December 31, 2007.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company's independent registered public accountants, Ernst & Young LLP, audited the financial statements included in this Annual Report on Form 10-K and have issued an audit report on the Company's internal control over financial reporting. The report on the audit of internal control over financial reporting appears below.
54
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders
Sonus Pharmaceuticals, Inc.
We have audited Sonus Pharmaceuticals, Inc.'s internal control over financial reporting as of December 31, 2007, based on criteria established in Internal ControlIntegrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Sonus Pharmaceuticals, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Sonus Pharmaceuticals, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Sonus Pharmaceuticals, Inc. as of December 31, 2007 and December 31, 2006, and the related statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2007 of Sonus Pharmaceuticals, Inc. and our report dated March 14, 2008 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP | ||
|
Seattle, Washington March 14, 2008 |
55
Not applicable.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
In compliance with Section 406 of the Sarbanes-Oxley Act of 2002 and the Nasdaq corporate governance listing standards, the Company has adopted a code of conduct that is applicable to all of the Company's employees and directors. Interested parties may request a copy of this code of conduct, free of charge, by delivering a written request addressed to the Chief Financial Officer, Sonus Pharmaceuticals, Inc., 1522 217th Place SE, Suite 100, Bothell, Washington 98021, or view it on the Company's website at www.sonuspharma.com. The Company will disclose any amendments to the code of conduct and any waivers from the code of conduct for directors and executive officers by posting such information on its website at www.sonuspharma.com.
The information required hereunder is incorporated by reference from our definitive Proxy Statement to be filed within 120 days of December 31, 2007 and delivered to stockholders in connection with our 2008 Annual Meeting of Stockholders.
ITEM 11. EXECUTIVE COMPENSATION
The information required hereunder is incorporated by reference from our definitive Proxy Statement to be filed within 120 days of December 31, 2007 and delivered to stockholders in connection with our 2008 Annual Meeting of Stockholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding our equity compensation plans as of December 31, 2007:
|
|
(a) |
(b) |
(c) |
||||
|---|---|---|---|---|---|---|---|
|
Plan category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a) |
||||
| Equity compensation plans approved by security holders(1) | 3,854,527 | $ | 4.79 | 5,253,071 | |||
| Equity compensation plans not approved by security holders(2) | 424,433 | $ | 5.04 | 57,581 | |||
| Total | 4,278,960 | 5,310,652 | |||||
- (1)
- Our 2000 Stock Incentive Plan was approved by security holders with 500,000 shares authorized under the plan. Stock options issued under the 2000 plan are generally granted at the fair market value on the date of grant and expire ten years from the date of grant. The plan also has an annual feature whereby an incremental number of shares equal to four percent of the Company's common stock outstanding as of December 31 of each year commencing December 31, 2000 are made available for issuance under the plan up to a lifetime maximum of five million shares. This lifetime maximum was reached in 2006. In 2007, Sonus shareholders approved a new incentive plan entitled the "2007 Performance Incentive Plan." Under the term of this plan the Company can issue up to 3,900,000 additional shares of the Company's common stock through the grant of stock
56
options and restricted stock. 5,213,520 shares were available for issuance as of December 31, 2007. The Company also had 39,551shares available at December 31, 2007 for issuance under its Employee Stock Purchase Plan. This plan expires in 2016.
- (2)
- Our 1999 Nonqualified Stock Incentive Plan (the "1999 Plan") is a broad-based plan for which shareholder approval was not required or obtained. A total of 900,000 shares are authorized under the 1999 Plan with 57,581 available for issuance as of December 31, 2007. Options to purchase 424,433 shares of common stock under the 1999 Plan were outstanding as of December 31, 2007 at a weighted average exercise price of $5.04. Stock options issued under the 1999 Plan are generally granted with an exercise price equal to fair market value on the date of grant, but in no event may be less than 85% of the then fair market value. Options under the 1999 Plan have various vesting schedules and expire ten years from the date of grant. The 1999 Plan also authorizes the issuance of restricted stock, although no restricted stock grants have been issued under the 1999 Plan. Shares underlying unexercised options that expire or are terminated become available again for future grants.
The remaining information required hereunder is incorporated by reference from our definitive Proxy Statement to be filed within 120 days of December 31, 2007 and delivered to stockholders in connection with its 2008 Annual Meeting of Stockholders.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required hereunder is incorporated by reference from our definitive Proxy Statement to be filed within 120 days of December 31, 2007 and delivered to stockholders in connection with our 2008 Annual Meeting of Stockholders.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required hereunder is incorporated by reference from our definitive Proxy Statement to be filed within 120 days of December 31, 2007 and delivered to stockholders in connection with its 2008 Annual Meeting of Stockholders.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
- (a)
- (1) Financial Statements
| Report of Independent Registered Public Accounting Firm | 31 | |
Balance Sheets as of December 31, 2007 and 2006 |
32 |
|
Statements of Operations for the years ended December 31, 2007, 2006, and 2005 |
33 |
|
Statements of Stockholders' Equity for the years ended December 31, 2007, 2006, and 2005 |
34 |
|
Statements of Cash Flows for the years ended December 31, 2007, 2006, and 2005 |
35 |
|
Notes to the Financial Statements |
36 |
- (2)
- All schedules are omitted because they are not required or the required information is included in the financial statements or notes thereto.
57
- (3)
- Exhibits
|
Exhibit No. |
Index to Exhibits Description |
Location |
||||
|---|---|---|---|---|---|---|
| Exhibit No. 2: Plan of Acquisition | ||||||
| 2.1 | Stock Purchase Agreement, dated November 3, 2004 | (15 | ) | |||
| 2.2 | Amended and Restated Stock Purchase Agreement, dated December 22, 2004. | (16 | ) | |||
Exhibit No. 3: Articles of Incorporation |
||||||
| 3.2 | Amended and Restated Certificate of Incorporation of the Company. | (1 | ) | |||
| 3.3 | Certificate of Amendment of Certificate of Incorporation of the Company. | (6 | ) | |||
| 3.4 | Second Amended and Restated Bylaws of the Company. | (26 | ) | |||
| 3.5 | Amended and Restated Certificate of Incorporation of the Company. | (13 | ) | |||
Exhibit No. 4: Instruments Defining the Rights of Security Holders |
||||||
| 4.1 | Specimen Certificate of Common Stock. | (1 | ) | |||
| 4.2 | Rights Agreement, dated as of August 23, 1996, between the Company and U.S. Stock Transfer Corporation. | (3 | ) | |||
| 4.3 | First Amendment to Rights Agreement, dated as of August 23, 1996, between the Company and U.S. Stock Transfer Corporation. | (12 | ) | |||
| 4.4 | Second Amendment to Amended and Restated Rights Agreement, dated August 10, 2006, between the Company and U.S. Stock Transfer Corporation, as Rights Agent. | (20 | ) | |||
Exhibit No. 10: Material Contracts |
||||||
|
Compensation Plans and Arrangements |
||||||
| 10.1 | Sonus Pharmaceuticals, Inc. Incentive Stock Option, Nonqualified Stock Option and Restricted Stock Purchase Plan1991 (the "1991 Plan"), as amended. | (1 | ) | |||
| 10.2 | Form of Incentive Stock Option Agreement pertaining to the 1991 Plan. | (1 | ) | |||
| 10.3 | Form of Nonqualified Stock Option Agreement pertaining to the 1991 Plan. | (1 | ) | |||
| 10.4 | Form of Restricted Stock Purchase Agreement pertaining to the 1991 Plan. | (1 | ) | |||
| 10.5 | Sonus Pharmaceuticals, Inc. 1995 Stock Option Plan for Directors (the "Director Plan"). | (1 | ) | |||
| 10.6 | Form of Stock Option Agreement pertaining to the Director Plan. | (1 | ) | |||
| 10.7 | 1999 Nonqualified Stock Incentive Plan (the "1999 Plan"). | (6 | ) | |||
| 10.8 | Form of Stock Option Agreement pertaining to the 1999 Plan. | (6 | ) | |||
| 10.9 | Form of Restricted Stock Purchase Agreement pertaining to the 1999 Plan. | (6 | ) | |||
| 10.10 | 2000 Stock Incentive Plan (the "2000 Plan"). | (8 | ) | |||
| 10.11 | Form of Stock Option Agreement pertaining to the 2000 Plan. | (8 | ) | |||
| 10.12 | Sonus Pharmaceuticals, Inc. Employee Stock Purchase Plan. | (2 | ) | |||
| 10.13 | Change in Control Agreement for Michael Martino, dated January 11, 2008. | (4 | ) | |||
| 10.14 | Change in Control Agreement for Alan Fuhrman, dated January 11, 2008. | (4 | ) | |||
| 10.15 | Amended and Restated Executive Compensation Program. | (17 | ) | |||
| 10.16 | Form of Performance Award under Executive Compensation Program. | (17 | ) | |||
| 10.17 | First Amendment to Sonus Pharmaceuticals, Inc. 2000 Stock Incentive Plan. | (21 | ) | |||
| 10.18 | Sonus Pharmaceuticals, Inc. Compensation Policy. | (22 | ) | |||
| 10.19 | Sonus Pharmaceuticals, Inc. Executive Compensation Program. | (22 | ) | |||
| 10.20 | Sonus Pharmaceuticals, Inc. 2007 Stock Performance Incentive Plan (the "2007 Plan"). | (23 | ) | |||
| 10.21 | Form of Stock Option Agreement pertaining to the 2007 Plan. | (24 | ) | |||
| 10.22 | Form of Restricted Stock Purchase Agreement pertaining to the 2007 Plan. | (24 | ) | |||
58
|
Other Material Contracts |
||||||
| 10.23 | Form of Indemnification Agreement for Officers and Directors of the Company. | (1 | ) | |||
| 10.24 | License Agreement by and between Nycomed Amersham AS and the Company dated August 31, 1999. | (7 | ) | |||
| 10.25 | License Agreement by and between Chugai Pharmaceutical Co. Ltd., Molecular Biosystems, Inc., and the Company, dated December 22, 2000.* | (9 | ) | |||
| 10.26 | Nycomed Assignment and Asset Transfer Agreement, dated August 3, 2001.* | (10 | ) | |||
| 10.27 | Supply Agreement dated January 22, 2002 between Indena SpA and Sonus Pharmaceuticals, Inc. | (11 | ) | |||
| 10.28 | First Amendment dated May 5, 2003 to Supply Agreement dated January 22, 2002 between Indena SpA and Sonus Pharmaceuticals, Inc. | (16 | ) | |||
| 10.29 | Manufacturing and Supply Agreement by and between the Company and Gensia Sicor Pharmaceutical Sales, Inc., dated June 26, 2002.* | (12 | ) | |||
| 10.30 | Securities Purchase Agreement, dated May 7, 2004. | (14 | ) | |||
| 10.31 | Registration Rights Agreement, dated May 7, 2004. | (14 | ) | |||
| 10.32 | Securities Purchase Agreement, dated August 15, 2005. | (18 | ) | |||
| 10.33 | Registration Rights Agreement, dated August 15, 2005. | (18 | ) | |||
| 10.34 | Collaboration and License Agreement by and between the Company and Bayer Schering Pharma AG, dated October 17, 2005.* | (19 | ) | |||
| 10.35 | Securities Purchase Agreement, dated October 17, 2005. | (19 | ) | |||
| 10.36 | Registration Rights Agreement, dated October 17, 2005. | (19 | ) | |||
| 10.37 | Lease Agreement dated November 21, 2006, between the Company and BMR-217TH Place LLC ("Lease"). | (5 | ) | |||
| 10.38 | Clinical Supply Agreement between the Company and Bayer Schering Pharma AG, dated June 1, 2006 | (5 | ) | |||
| 10.39 | Placement Agency Agreement, dated April 27, 2006, by and among the Company, Needham & Company, LLC, Ziegel & Company, L.P. and ThinkEquity Partners LLC. | (25 | ) | |||
| 10.40 | Form of Purchase Agreement between the Company and each of certain investors. | (25 | ) | |||
| 10.41 | First Amendment to Lease, dated August 17, 2007. | (26 | ) | |||
Exhibit No. 23: Consents of Experts and Counsel |
||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm. | (26 | ) | |||
| 24.1 | Power of Attorney (included on the Signature Page of this Annual Report on Form 10-K). | (26 | ) | |||
Certifications |
||||||
| 31.1 | Certification of President and Chief Executive Officer pursuant to Rule 13a-14(a) or 15d-14(a). | (26 | ) | |||
| 31.2 | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) or 15d-14(a). | (26 | ) | |||
| 32.1 | Certification of President and Chief Executive Officer pursuant to Rule 13a-14(b) or 15d-14(b). | (26 | ) | |||
| 32.2 | Certification of the Chief Financial Officer pursuant to Rule 13a-14(b) or 15d-14(b). | (26 | ) | |||
- (1)
- Incorporated by reference to the Company's Registration Statement on Form S-1, Reg. No. 33-96112.
59
- (2)
- Incorporated
by reference to the Company's Registration Statement on Form S-1, Reg. No. 33-80623.
- (3)
- Incorporated
by reference to the Company's Registration Statement on Form 8-A, dated August 23, 1996.
- (4)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed January 17, 2008.
- (5)
- Incorporated
by reference to the Company's Annual Report on Form 10-K for the period ended December 31, 2006.
- (6)
- Incorporated
by reference to the Company's Quarterly Report on Form 10-Q for the quarterly period ended March 31, 1999 (File
No. 000-21243; Film No. 99618950).
- (7)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed October 14, 1999 (File No. 000-21243; Film
No. 99728284).
- (8)
- Incorporated
by reference to the Company's Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2000 (File No. 000-21243;
Film No. 698483).
- (9)
- Incorporated
by reference to the Company's Annual Report on Form 10-KA for the period ended December 31, 2000 (File No. 000-21243; Film
No. 1562780).
- (10)
- Incorporated
by reference to the Company's Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2001 (File
No. 000-21243; Film No. 1783325).
- (11)
- Incorporated
by reference to the Company's Registration Statement on Form S-3 filed February 8, 2002.
- (12)
- Incorporated
by reference to the Company's filing on Form 8-A12G/A dated July 25, 2002.
- (13)
- Incorporated
by reference to the Company's Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2004.
- (14)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed May 13, 2004.
- (15)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed September 20, 2004.
- (16)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed November 8, 2004.
- (17)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed January 4, 2005.
- (18)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed August 17, 2005.
- (19)
- Incorporated
by reference to the Company's Annual Report on Form 10-K for the period ended December 31, 2005.
- (20)
- Incorporated
by reference to the Company's Current Report on Form 8-K-A/A filed August 10, 2006.
- (21)
- Incorporated
by reference to the Company's Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2006.
- (22)
- Incorporated
by reference to the Company's Current Report on Form 8-K filed December 15, 2006.
- (23)
- Incorporated
by reference to the Company's Definitive Proxy Statement on Schedule 14A, filed April 3, 2007.
- (24)
- Incorporated by reference to the Company's Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2007.
60
- (25)
- Incorporated
by reference to the Company's Current Report on Form 8-K, filed May 1, 2006.
- (26)
- Filed
herewith.
- *
- Portions of this exhibit are omitted and were filed separately with the Secretary of the Commission pursuant to the Registrant's application requesting confidential treatment under Rule 24b-2 of the Exchange Act of the Securities Exchange Act of 1934.
61
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized, in the City of Bothell, State of Washington, on March 14, 2008.
| SONUS PHARMACEUTICALS, INC. | |||
Dated: March 14, 2008 |
By: |
/s/ MICHAEL A. MARTINO Michael A. Martino President, Chief Executive Officer and Director (Principal Executive Officer) |
|
We, the undersigned directors and officers of Sonus Pharmaceuticals, Inc., do hereby constitute and appoint Michael A. Martino and Alan Fuhrman, or either of them, our true and lawful attorneys and agents, with full powers of substitution to do any and all acts and things in our name and on behalf in our capacities as directors and officers and to execute any and all instruments for us and in our names in the capacities indicated below, which said attorneys and agents may deem necessary or advisable to enable said corporation to comply with the Securities Exchange Act of 1934, as amended, and any rules, regulations and requirements of the Securities and Exchange Commission, in connection with this Annual Report on Form 10-K, including specifically but without limitation, power and authority to sign for us or any of us in our names in the capacities indicated below, any and all amendments thereto; and we do hereby ratify and confirm all that said attorneys and agents, shall do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
/s/ MICHAEL A. MARTINO Michael A. Martino |
President, Chief Executive Officer and Director (Principal Executive Officer) | March 14, 2008 | ||
|
/s/ ALAN FUHRMAN Alan Fuhrman |
Senior Vice President, Chief Financial Officer (Principal Financial Officer) (Principal Accounting Officer) |
March 14, 2008 |
||
|
/s/ MICHELLE BURRIS Michelle Burris |
Director |
March 14, 2008 |
||
|
/s/ GEORGE W. DUNBAR, JR. George W. Dunbar, Jr. |
Director |
March 14, 2008 |
||
|
/s/ ROBERT E. IVY Robert E. Ivy |
Director, Chairman of the Board of Directors |
March 14, 2008 |
||
|
/s/ DWIGHT WINSTEAD Dwight Winstead |
Director |
March 14, 2008 |
62
Sonus Pharmaceuticals, Inc. Table of Contents
PART I
-
ITEM 2. PROPERTIES
ITEM 3. LEGAL PROCEEDINGS
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN* Among SONUS Pharmaceuticals, Inc., The NASDAQ Composite Index And The NASDAQ Pharmaceutical Index
-
ITEM 6. SELECTED FINANCIAL DATA
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Sonus Pharmaceuticals, Inc. Balance Sheets
Sonus Pharmaceuticals, Inc. Statements of Operations
Sonus Pharmaceuticals, Inc. Statements of Stockholders' Equity
Sonus Pharmaceuticals, Inc. Statements of Cash Flows
Sonus Pharmaceuticals, Inc. Notes to Financial Statements
-
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
ITEM 9A. CONTROLS AND PROCEDURES
Report of Independent Registered Public Accounting Firm
PART III
-
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
ITEM 11. EXECUTIVE COMPENSATION
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
SIGNATURES