EXHIBIT 99.2
Published on March 11, 2009
Exhibit 99.2

| OncoGenex Pharmaceuticals, Inc. (NASDAQ: OGXI) Financial Results For Fourth Quarter and Fiscal Year End 2008 and Outlook for 2009 Committed to the development of new therapies that address unmet needs in the treatment of cancer |

| Forward-Looking Statements This presentation contains forward-looking statements, including statements concerning anticipated clinical development activities, the potential benefits of product candidates and anticipated market opportunities. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. These statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, among others, the possibility that interim clinical trial results will not be maintained or will become less substantial as patient survival follow up continues, risks that clinical trials will not be successful or confirm earlier clinical trial results, risks associated with obtaining funding from third parties, risks related to the timing and costs of clinical trials and the receipt of regulatory approvals, and the risk factors set forth in the company's filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for fiscal year 2008. The company undertakes no obligation to update the forward-looking statements contained herein or to reflect events or circumstances occurring after the date hereof. |

| Content for Today's Conference Update on current cash position and financial results Summarize the results of our phase 2 clinical trials for OGX-011 Present an update on our clinical development strategy for OGX-011 marketing approval Provide an update on our other product candidates which are currently under clinical investigation Review our objectives for 2009 |
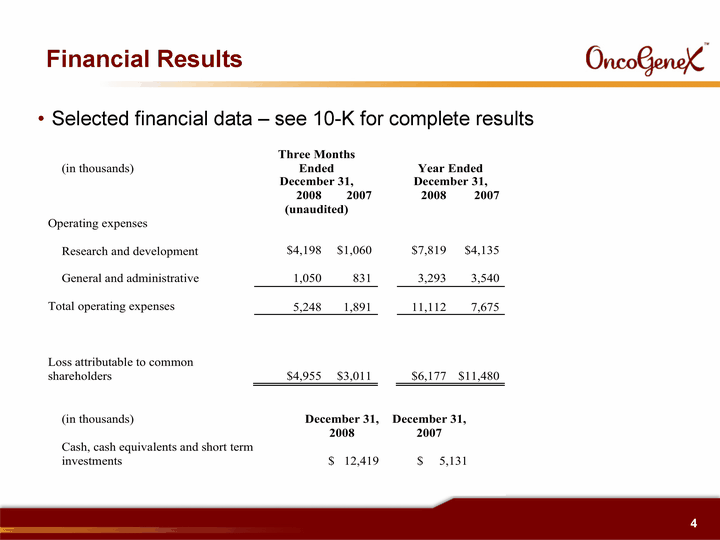
| Financial Results Selected financial data - see 10-K for complete results |
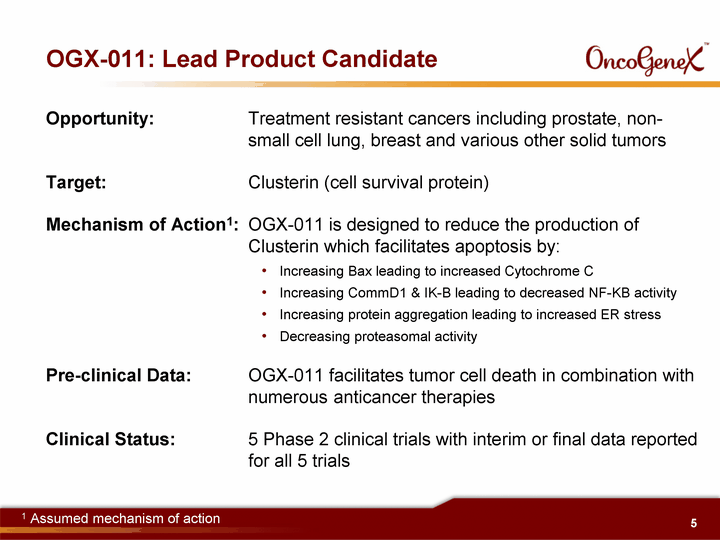
| OGX-011: Lead Product Candidate Opportunity: Treatment resistant cancers including prostate, non- small cell lung, breast and various other solid tumors Target: Clusterin (cell survival protein) Mechanism of Action1: OGX-011 is designed to reduce the production of Clusterin which facilitates apoptosis by: Increasing Bax leading to increased Cytochrome C Increasing CommD1 & IK-B leading to decreased NF-KB activity Increasing protein aggregation leading to increased ER stress Decreasing proteasomal activity Pre-clinical Data: OGX-011 facilitates tumor cell death in combination with numerous anticancer therapies Clinical Status: 5 Phase 2 clinical trials with interim or final data reported for all 5 trials 1 Assumed mechanism of action |
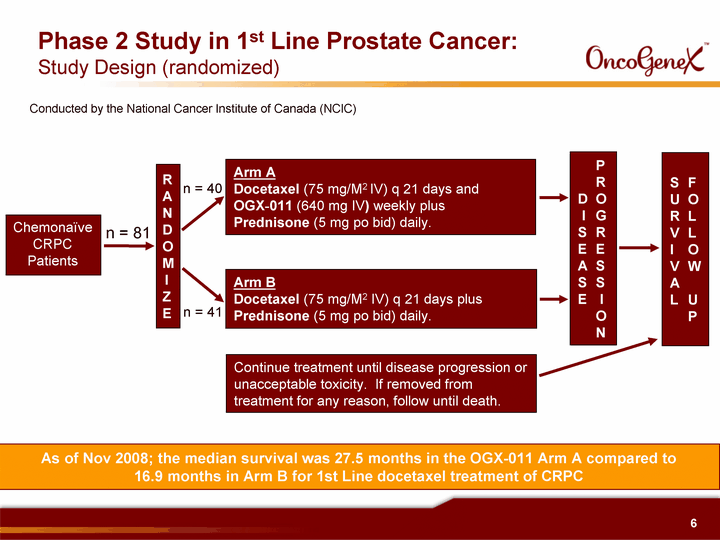
| Chemonaive CRPC Patients Phase 2 Study in 1st Line Prostate Cancer: Study Design (randomized) R A N D O M I Z E Arm A Docetaxel (75 mg/M2 IV) q 21 days and OGX^011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily. Arm B Docetaxel (75 mg/M2 IV) q 21 days plus Prednisone (5 mg po bid) daily. Continue treatment until disease progression or unacceptable toxicity. If removed from treatment for any reason, follow until death. P R D O I G S R E E A S S S E I O N S F U O R L V L I O V W A L U P n = 81 n = 40 n = 41 Conducted by the National Cancer Institute of Canada (NCIC) As of Nov 2008; the median survival was 27.5 months in the OGX-011 Arm A compared to 16.9 months in Arm B for 1st Line docetaxel treatment of CRPC |
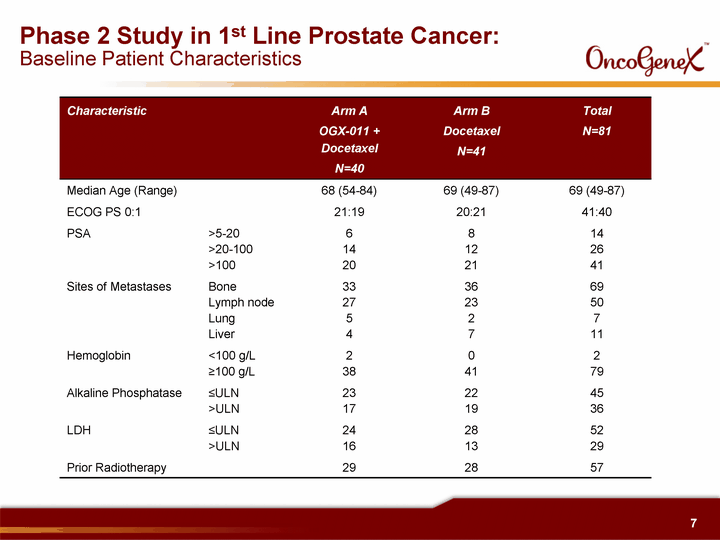
| Phase 2 Study in 1st Line Prostate Cancer: Baseline Patient Characteristics Characteristic Arm A OGX-011 + Docetaxel N=40 Arm B Docetaxel N=41 Total N=81 Median Age (Range) 68 (54-84) 69 (49-87) 69 (49-87) ECOG PS 0:1 21:19 20:21 41:40 PSA >5-20 >20-100 >100 6 14 20 8 12 21 14 26 41 Sites of Metastases Bone Lymph node Lung Liver 33 27 5 4 36 23 2 7 69 50 7 11 Hemoglobin <100 g/L ^100 g/L 2 38 0 41 2 79 Alkaline Phosphatase ^ULN >ULN 23 17 22 19 45 36 LDH ^ULN >ULN 24 16 28 13 52 29 Prior Radiotherapy 29 28 57 |
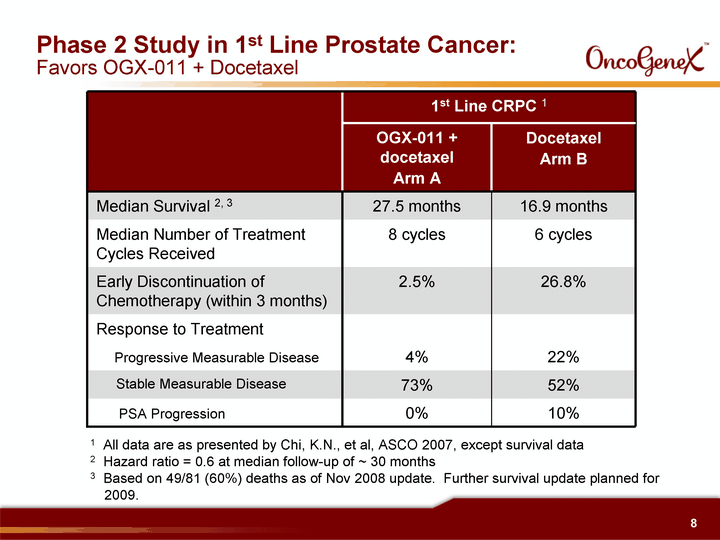
| Phase 2 Study in 1st Line Prostate Cancer: Favors OGX-011 + Docetaxel 1st Line CRPC 1 1st Line CRPC 1 OGX-011 + docetaxel Arm A Docetaxel Arm B Median Survival 2, 3 27.5 months 16.9 months Median Number of Treatment Cycles Received 8 cycles 6 cycles Early Discontinuation of Chemotherapy (within 3 months) 2.5% 26.8% Response to Treatment Progressive Measurable Disease 4% 22% Stable Measurable Disease 73% 52% PSA Progression 0% 10% 1 All data are as presented by Chi, K.N., et al, ASCO 2007, except survival data 2 Hazard ratio = 0.6 at median follow-up of ~ 30 months 3 Based on 49/81 (60%) deaths as of Nov 2008 update. Further survival update planned for 2009. |
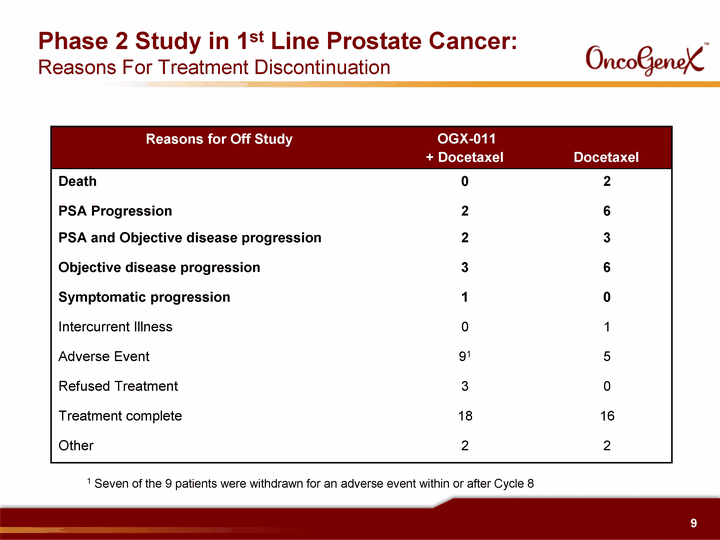
| Phase 2 Study in 1st Line Prostate Cancer: Reasons For Treatment Discontinuation Reasons for Off Study OGX-011 + Docetaxel Docetaxel OGX-011 + Docetaxel Docetaxel Death 0 2 PSA Progression 2 6 PSA and Objective disease progression 2 3 Objective disease progression 3 6 Symptomatic progression 1 0 Intercurrent Illness 0 1 Adverse Event 91 5 Refused Treatment 3 0 Treatment complete 18 16 Other 2 2 1 Seven of the 9 patients were withdrawn for an adverse event within or after Cycle 8 |
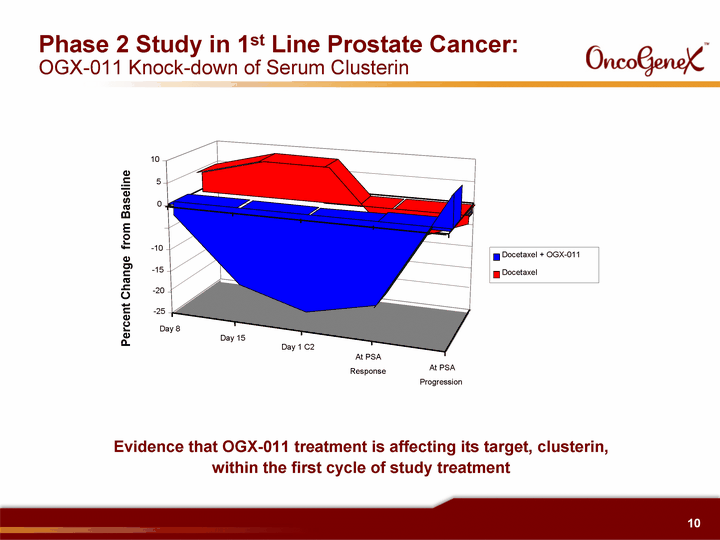
| Phase 2 Study in 1st Line Prostate Cancer: OGX-011 Knock-down of Serum Clusterin Percent Change from Baseline Day 8 Day 15 Day 1 C2 At PSA Response At PSA Progression - -25 - -20 - -15 - -10 0 5 10 Docetaxel + OGX-011 Docetaxel Evidence that OGX-011 treatment is affecting its target, clusterin, within the first cycle of study treatment |
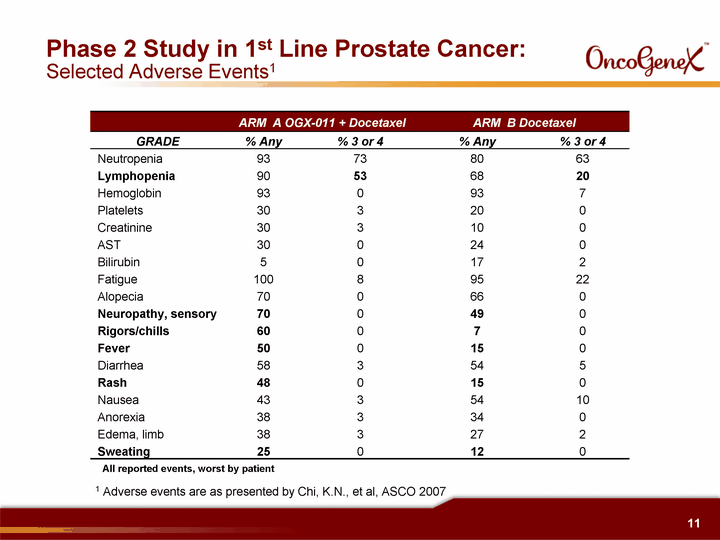
| Phase 2 Study in 1st Line Prostate Cancer: Selected Adverse Events1 ARM A OGX-011 + Docetaxel ARM A OGX-011 + Docetaxel ARM B Docetaxel ARM B Docetaxel GRADE % Any % 3 or 4 % Any % 3 or 4 Neutropenia 93 73 80 63 Lymphopenia 90 53 68 20 Hemoglobin 93 0 93 7 Platelets 30 3 20 0 Creatinine 30 3 10 0 AST 30 0 24 0 Bilirubin 5 0 17 2 Fatigue 100 8 95 22 Alopecia 70 0 66 0 Neuropathy, sensory 70 0 49 0 Rigors/chills 60 0 7 0 Fever 50 0 15 0 Diarrhea 58 3 54 5 Rash 48 0 15 0 Nausea 43 3 54 10 Anorexia 38 3 34 0 Edema, limb 38 3 27 2 Sweating 25 0 12 0 All reported events, worst by patient 1 Adverse events are as presented by Chi, K.N., et al, ASCO 2007 |
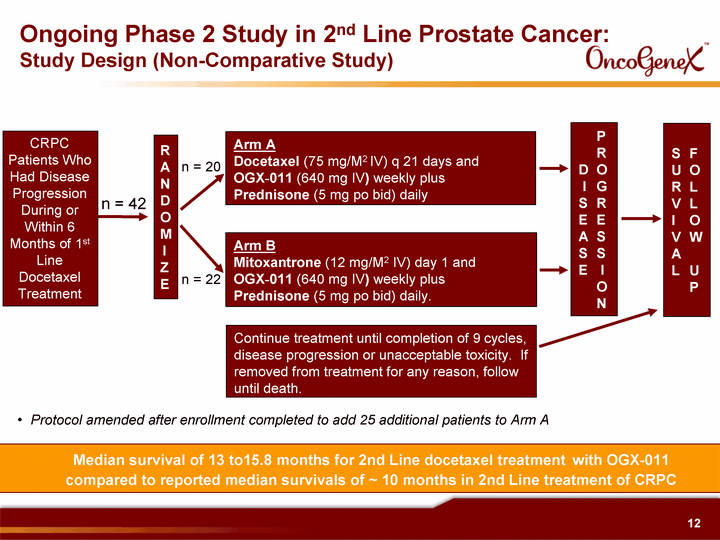
| Median survival of 13 to15.8 months for 2nd Line docetaxel treatment with OGX-011 compared to reported median survivals of ~ 10 months in 2nd Line treatment of CRPC Ongoing Phase 2 Study in 2nd Line Prostate Cancer: Study Design (Non-Comparative Study) R A N D O M I Z E Arm A Docetaxel (75 mg/M2 IV) q 21 days and OGX^011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily Arm B Mitoxantrone (12 mg/M2 IV) day 1 and OGX^011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily. Continue treatment until completion of 9 cycles, disease progression or unacceptable toxicity. If removed from treatment for any reason, follow until death. P R D O I G S R E E A S S S E I O N S F U O R L V L I O V W A L U P n = 42 n = 20 n = 22 Protocol amended after enrollment completed to add 25 additional patients to Arm A CRPC Patients Who Had Disease Progression During or Within 6 Months of 1st Line Docetaxel Treatment |
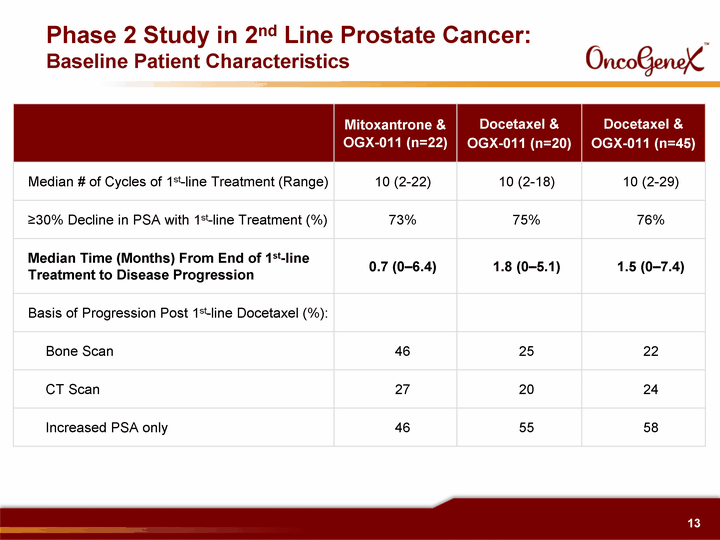
| Phase 2 Study in 2nd Line Prostate Cancer: Baseline Patient Characteristics Mitoxantrone & OGX-011 (n=22) Docetaxel & OGX-011 (n=20) Docetaxel & OGX-011 (n=45) Median # of Cycles of 1st-line Treatment (Range) 10 (2-22) 10 (2-18) 10 (2-29) ^30% Decline in PSA with 1st-line Treatment (%) 73% 75% 76% Median Time (Months) From End of 1st-line Treatment to Disease Progression 0.7 (0-6.4) 1.8 (0-5.1) 1.5 (0-7.4) Basis of Progression Post 1st-line Docetaxel (%): Bone Scan 46 25 22 CT Scan 27 20 24 Increased PSA only 46 55 58 |
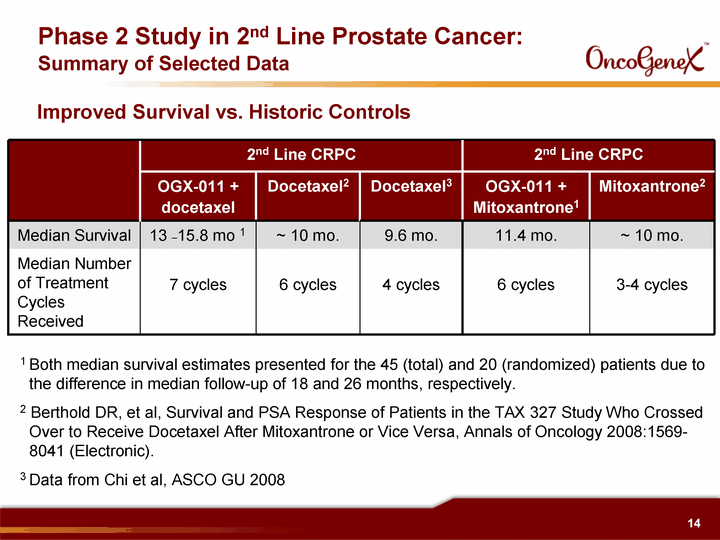
| Phase 2 Study in 2nd Line Prostate Cancer: Summary of Selected Data 2nd Line CRPC 2nd Line CRPC 2nd Line CRPC 2nd Line CRPC 2nd Line CRPC OGX-011 + docetaxel Docetaxel2 Docetaxel3 OGX-011 + Mitoxantrone1 Mitoxantrone2 Median Survival 13 -15.8 mo 1 ~ 10 mo. 9.6 mo. 11.4 mo. ~ 10 mo. Median Number of Treatment Cycles Received 7 cycles 6 cycles 4 cycles 6 cycles 3-4 cycles 1 Both median survival estimates presented for the 45 (total) and 20 (randomized) patients due to the difference in median follow-up of 18 and 26 months, respectively. 2 Berthold DR, et al, Survival and PSA Response of Patients in the TAX 327 Study Who Crossed Over to Receive Docetaxel After Mitoxantrone or Vice Versa, Annals of Oncology 2008:1569- 8041 (Electronic). 3 Data from Chi et al, ASCO GU 2008 Improved Survival vs. Historic Controls |
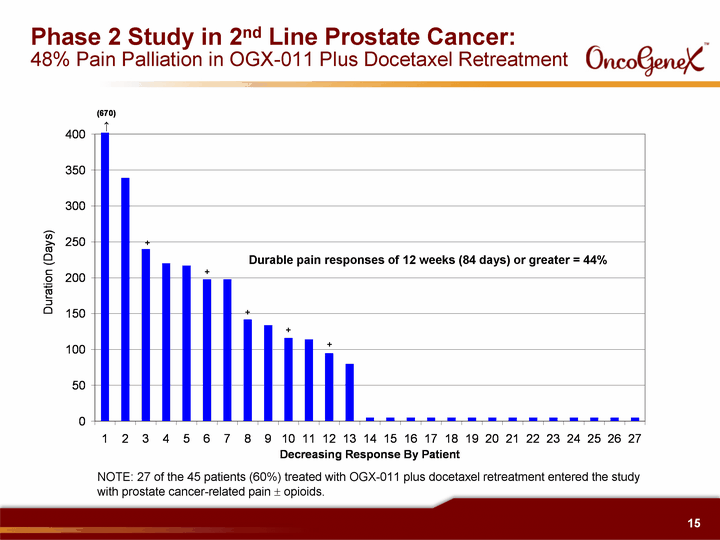
| Phase 2 Study in 2nd Line Prostate Cancer: 48% Pain Palliation in OGX-011 Plus Docetaxel Retreatment Durable pain responses of 12 weeks (84 days) or greater = 44% NOTE: 27 of the 45 patients (60%) treated with OGX-011 plus docetaxel retreatment entered the study with prostate cancer-related pain ? opioids. |
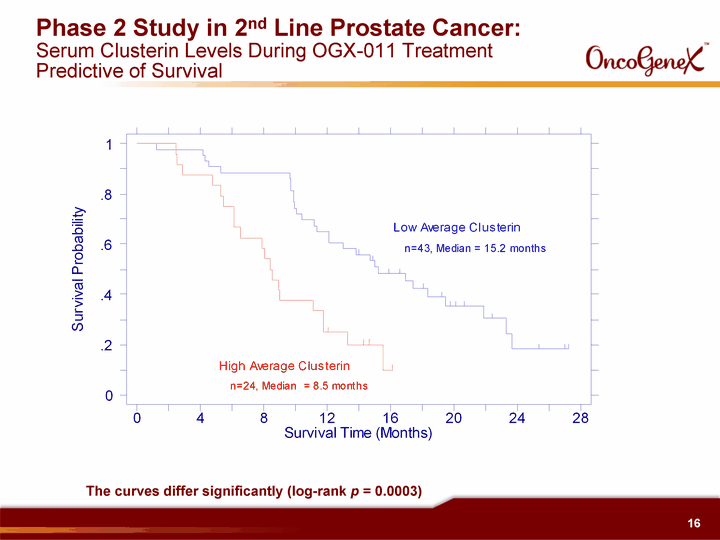
| Phase 2 Study in 2nd Line Prostate Cancer: Serum Clusterin Levels During OGX-011 Treatment Predictive of Survival The curves differ significantly (log-rank p = 0.0003) |
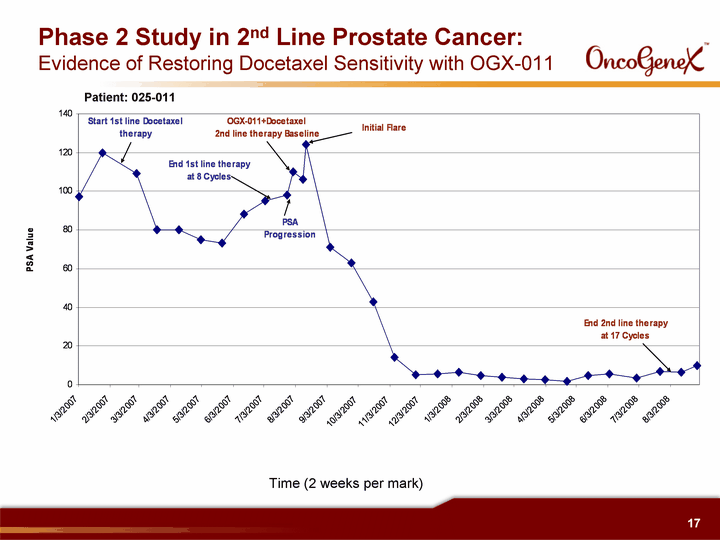
| Time (2 weeks per mark) Phase 2 Study in 2nd Line Prostate Cancer: Evidence of Restoring Docetaxel Sensitivity with OGX-011 Patient: 025-011 |
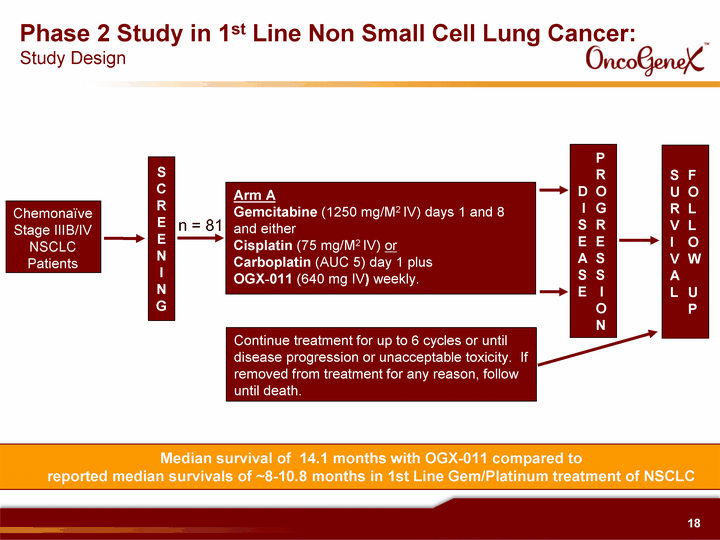
| Chemonaive Stage IIIB/IV NSCLC Patients Phase 2 Study in 1st Line Non Small Cell Lung Cancer: Study Design S C R E E N I N G Arm A Gemcitabine (1250 mg/M2 IV) days 1 and 8 and either Cisplatin (75 mg/M2 IV) or Carboplatin (AUC 5) day 1 plus OGX^011 (640 mg IV) weekly. Continue treatment for up to 6 cycles or until disease progression or unacceptable toxicity. If removed from treatment for any reason, follow until death. P R D O I G S R E E A S S S E I O N S F U O R L V L I O V W A L U P n = 81 Median survival of 14.1 months with OGX-011 compared to reported median survivals of ~8-10.8 months in 1st Line Gem/Platinum treatment of NSCLC |
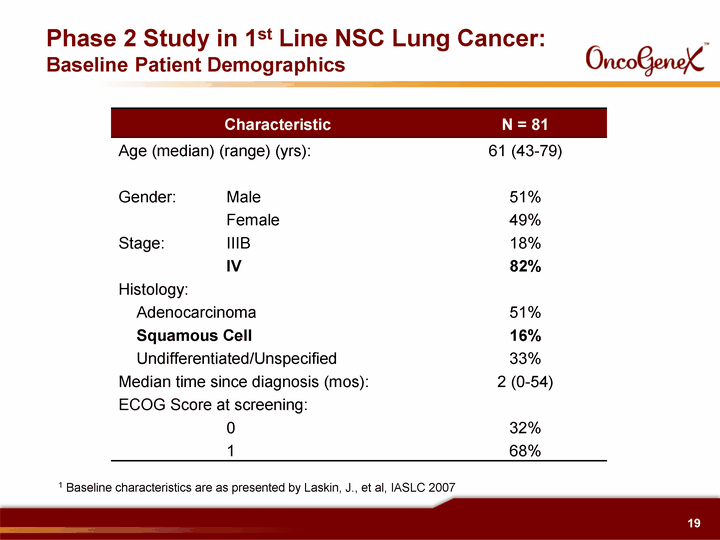
| Characteristic N = 81 Age (median) (range) (yrs): 61 (43-79) Gender: Male 51% Female 49% Stage: IIIB 18% IV 82% Histology: Adenocarcinoma 51% Squamous Cell 16% Undifferentiated/Unspecified 33% Median time since diagnosis (mos): 2 (0-54) ECOG Score at screening: 0 32% 1 68% Phase 2 Study in 1st Line NSC Lung Cancer: Baseline Patient Demographics 1 Baseline characteristics are as presented by Laskin, J., et al, IASLC 2007 |
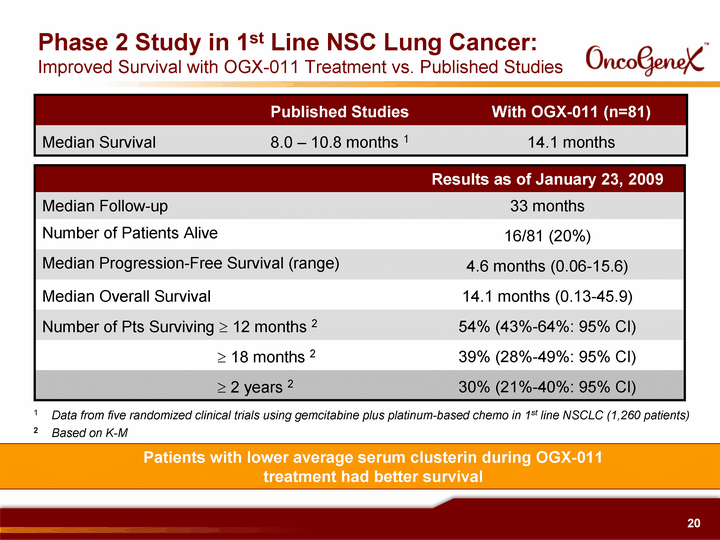
| Phase 2 Study in 1st Line NSC Lung Cancer: Improved Survival with OGX-011 Treatment vs. Published Studies Results as of January 23, 2009 Median Follow-up 33 months Number of Patients Alive 16/81 (20%) Median Progression-Free Survival (range) 4.6 months (0.06-15.6) Median Overall Survival 14.1 months (0.13-45.9) Number of Pts Surviving ? 12 months 2 54% (43%-64%: 95% CI) ? 18 months 2 39% (28%-49%: 95% CI) ? 2 years 2 30% (21%-40%: 95% CI) Published Studies With OGX-011 (n=81) Median Survival 8.0 - 10.8 months 1 14.1 months 1 Data from five randomized clinical trials using gemcitabine plus platinum-based chemo in 1st line NSCLC (1,260 patients) 2 Based on K-M Patients with lower average serum clusterin during OGX-011 treatment had better survival |
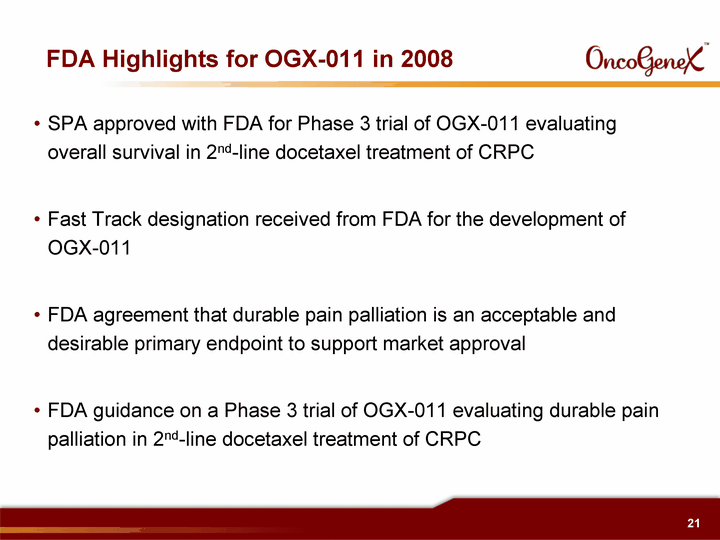
| FDA Highlights for OGX-011 in 2008 SPA approved with FDA for Phase 3 trial of OGX-011 evaluating overall survival in 2nd-line docetaxel treatment of CRPC Fast Track designation received from FDA for the development of OGX-011 FDA agreement that durable pain palliation is an acceptable and desirable primary endpoint to support market approval FDA guidance on a Phase 3 trial of OGX-011 evaluating durable pain palliation in 2nd-line docetaxel treatment of CRPC |

| Clinical Development Strategy for Approval Clinical Development Strategy is to obtain the following indication: OGX-011 in combination with docetaxel chemotherapy is indicated for the treatment of metastatic CRPC There are three possible Phase 3 trials that would provide evidence of safety and efficacy for OGX-011 in patients with metastatic CRPC. OGX-011-11: Primary endpoint is survival in 1st-line docetaxel chemotherapy OGX-011-08: Primary endpoint is survival in 2nd-line docetaxel chemotherapy OGX-011-10: Primary endpoint is durable pain palliation in 2nd-line docetaxel chemotherapy Selecting two of the above Phase 3 trials will be discussed with FDA |
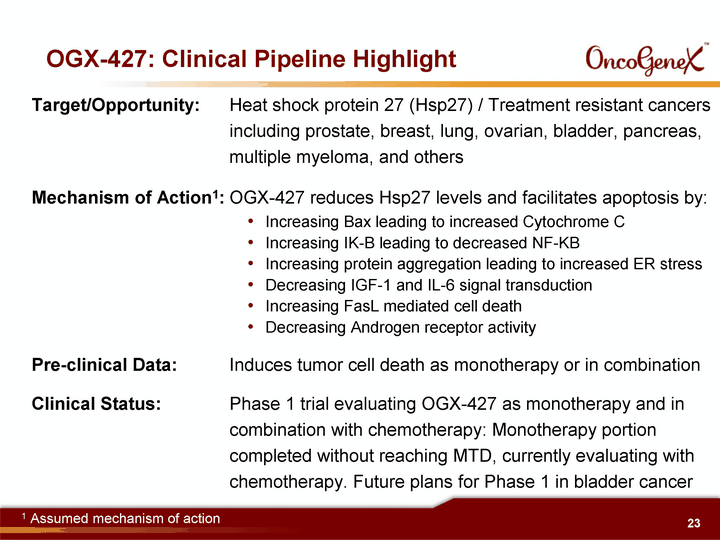
| OGX-427: Clinical Pipeline Highlight Target/Opportunity: Heat shock protein 27 (Hsp27) / Treatment resistant cancers including prostate, breast, lung, ovarian, bladder, pancreas, multiple myeloma, and others Mechanism of Action1: OGX-427 reduces Hsp27 levels and facilitates apoptosis by: Increasing Bax leading to increased Cytochrome C Increasing IK-B leading to decreased NF-KB Increasing protein aggregation leading to increased ER stress Decreasing IGF-1 and IL-6 signal transduction Increasing FasL mediated cell death Decreasing Androgen receptor activity Pre-clinical Data: Induces tumor cell death as monotherapy or in combination Clinical Status: Phase 1 trial evaluating OGX-427 as monotherapy and in combination with chemotherapy: Monotherapy portion completed without reaching MTD, currently evaluating with chemotherapy. Future plans for Phase 1 in bladder cancer 1 Assumed mechanism of action |
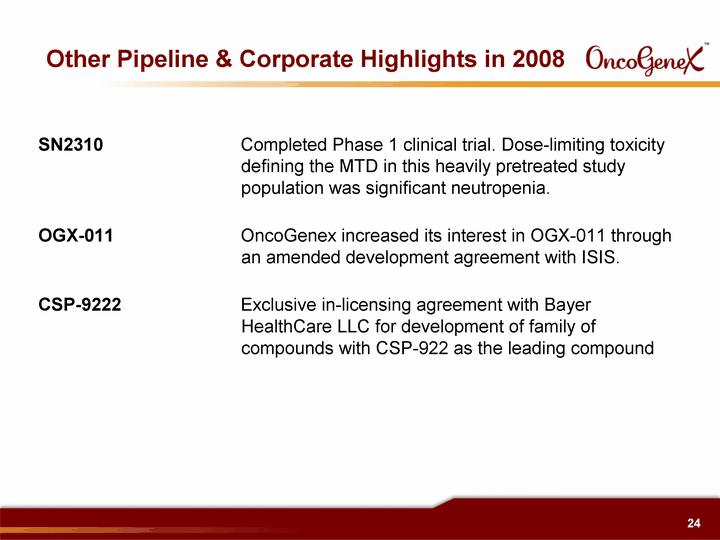
| Other Pipeline & Corporate Highlights in 2008 SN2310 Completed Phase 1 clinical trial. Dose-limiting toxicity defining the MTD in this heavily pretreated study population was significant neutropenia. OGX-011 OncoGenex increased its interest in OGX-011 through an amended development agreement with ISIS. CSP-9222 Exclusive in-licensing agreement with Bayer HealthCare LLC for development of family of compounds with CSP-922 as the leading compound |
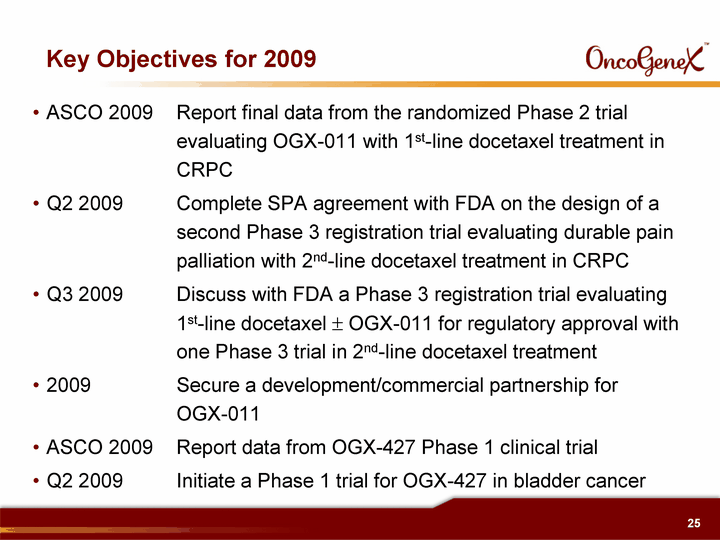
| Key Objectives for 2009 ASCO 2009 Report final data from the randomized Phase 2 trial evaluating OGX-011 with 1st-line docetaxel treatment in CRPC Q2 2009 Complete SPA agreement with FDA on the design of a second Phase 3 registration trial evaluating durable pain palliation with 2nd-line docetaxel treatment in CRPC Q3 2009 Discuss with FDA a Phase 3 registration trial evaluating 1st-line docetaxel ? OGX-011 for regulatory approval with one Phase 3 trial in 2nd-line docetaxel treatment 2009 Secure a development/commercial partnership for OGX-011 ASCO 2009 Report data from OGX-427 Phase 1 clinical trial Q2 2009 Initiate a Phase 1 trial for OGX-427 in bladder cancer |

| OncoGenex Pharmaceuticals, Inc. (NASDAQ: OGXI) Committed to the development of new therapies that address unmet needs in the treatment of cancer |