EX-99.1
Published on September 13, 2019

A multicenter, double-blind, randomized, placebo-controlled phase 2b trial of cytisinicline in adult smokers Nides M.1, Rigotti N.2, Benowitz N.3, Cain D.4, Clarke A.4, Jacobs C.4 1 Los Angeles Clinical Trials, Burbank, United States 2 Massachusetts General Hospital/Harvard Medical School, Boston, United States 3 University of California San Francisco, San Francisco, United States 4 Achieve Life Sciences, Inc., Seattle, United States Exhibit 99.1
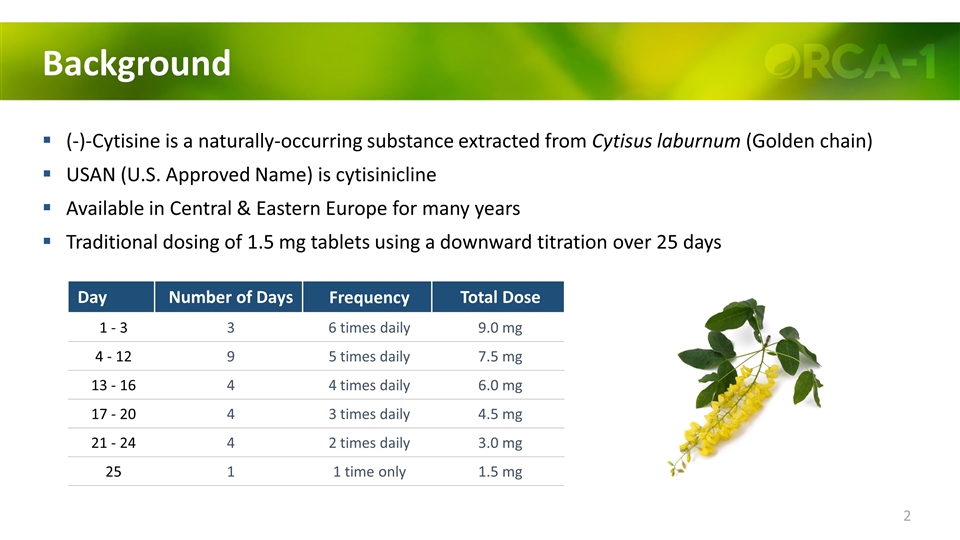
Background (-)-Cytisine is a naturally-occurring substance extracted from Cytisus laburnum (Golden chain) USAN (U.S. Approved Name) is cytisinicline Available in Central & Eastern Europe for many years Traditional dosing of 1.5 mg tablets using a downward titration over 25 days Day Number of Days Frequency Total Dose 1 - 3 3 6 times daily 9.0 mg 4 - 12 9 5 times daily 7.5 mg 13 - 16 4 4 times daily 6.0 mg 17 - 20 4 3 times daily 4.5 mg 21 - 24 4 2 times daily 3.0 mg 25 1 1 time only 1.5 mg

Objectives Evaluate Safety, efficacy & compliance Cytisinicline versus placebo Different doses/dosing schedules 25 days Optimize Dose/dosing schedule for Phase 3 trials

Overview Brief Methods Smokers of ≥10 cigarettes/day & expired air CO ≥10 ppm Standardized behavioral support 25 days’ treatment and follow-up to 8 weeks Daily diary of cigarettes smoked and regular assessments of CO-verified abstinence 8 trial centers in the United States
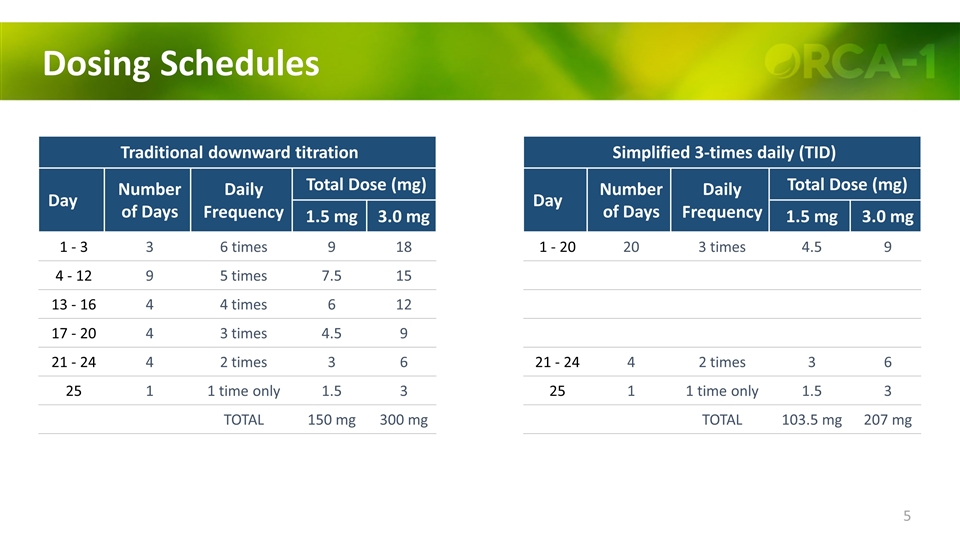
Dosing Schedules Traditional downward titration Day Number of Days Daily Frequency Total Dose (mg) 1.5 mg 3.0 mg 1 - 3 3 6 times 9 18 4 - 12 9 5 times 7.5 15 13 - 16 4 4 times 6 12 17 - 20 4 3 times 4.5 9 21 - 24 4 2 times 3 6 25 1 1 time only 1.5 3 TOTAL 150 mg 300 mg Simplified 3-times daily (TID) Day Number of Days Daily Frequency Total Dose (mg) 1.5 mg 3.0 mg 1 - 20 20 3 times 4.5 9 21 - 24 4 2 times 3 6 25 1 1 time only 1.5 3 TOTAL 103.5 mg 207 mg
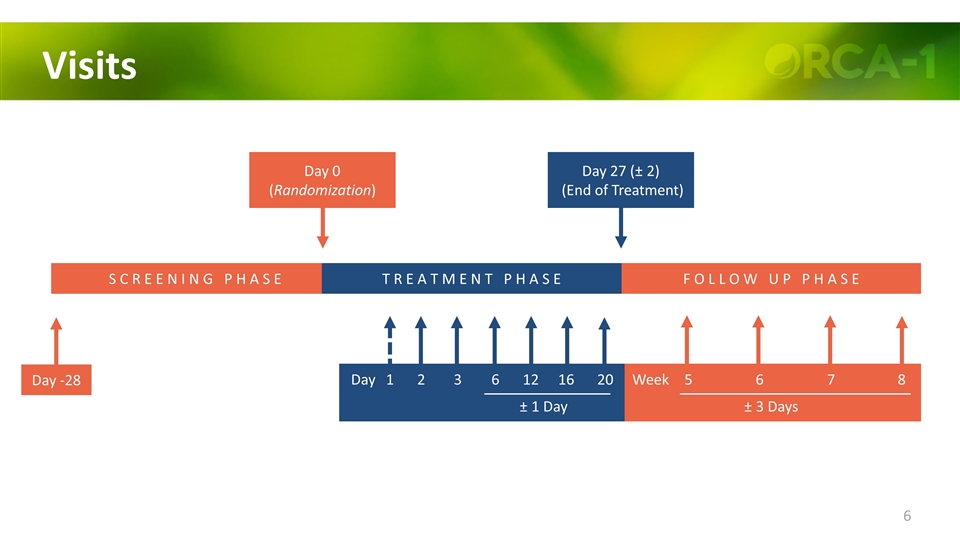
Visits Day -28 S C R E E N I N G P H A S E F O L L O W U P P H A S E Day 0 (Randomization) Day 27 (± 2) (End of Treatment) 1 2 3 6 12 16 Day 5 6 7 8 Week ± 3 Days T R E A T M E N T P H A S E 20 ± 1 Day
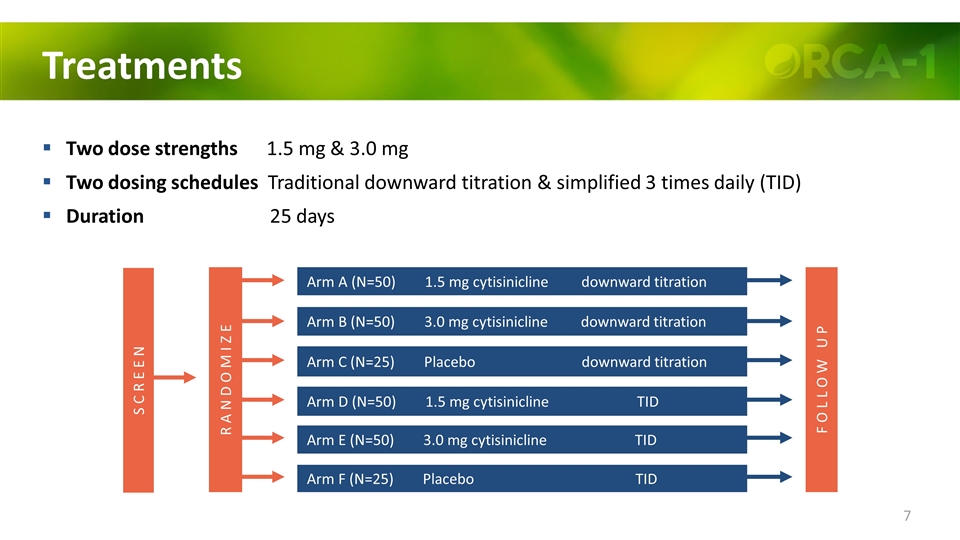
Treatments Two dose strengths 1.5 mg & 3.0 mg Two dosing schedules Traditional downward titration & simplified 3 times daily (TID) Duration 25 days Arm A (N=50) 1.5 mg cytisinicline downward titration Arm B (N=50) 3.0 mg cytisinicline downward titration Arm C (N=25) Placebo downward titration Arm D (N=50) 1.5 mg cytisinicline TID Arm E (N=50) 3.0 mg cytisinicline TID Arm F (N=25) Placebo TID R A N D O M I Z E F O L L O W U P S C R E E N
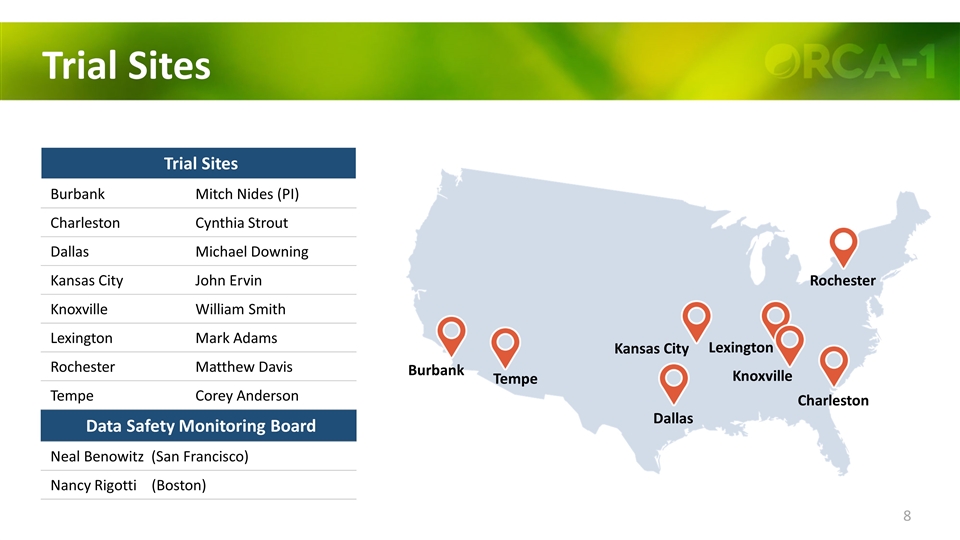
Trial Sites Tempe Burbank Dallas Kansas City Lexington Knoxville Charleston Rochester Trial Sites Burbank Mitch Nides (PI) Charleston Cynthia Strout Dallas Michael Downing Kansas City John Ervin Knoxville William Smith Lexington Mark Adams Rochester Matthew Davis Tempe Corey Anderson Data Safety Monitoring Board Neal Benowitz (San Francisco) Nancy Rigotti (Boston)
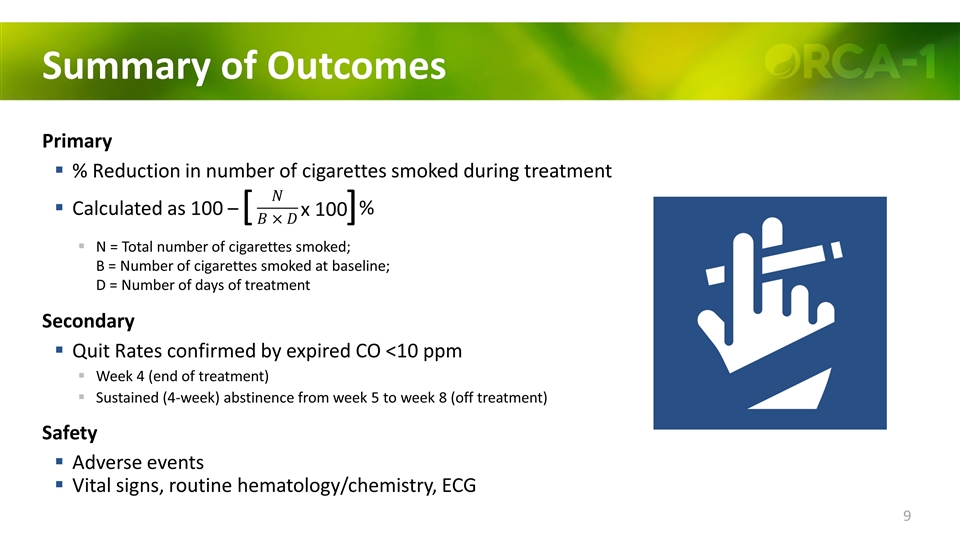
Summary of Outcomes Primary % Reduction in number of cigarettes smoked during treatment Calculated as 100 – N = Total number of cigarettes smoked; B = Number of cigarettes smoked at baseline; D = Number of days of treatment Secondary Quit Rates confirmed by expired CO <10 ppm Week 4 (end of treatment) Sustained (4-week) abstinence from week 5 to week 8 (off treatment) Safety Adverse events Vital signs, routine hematology/chemistry, ECG ] [ x 100 %
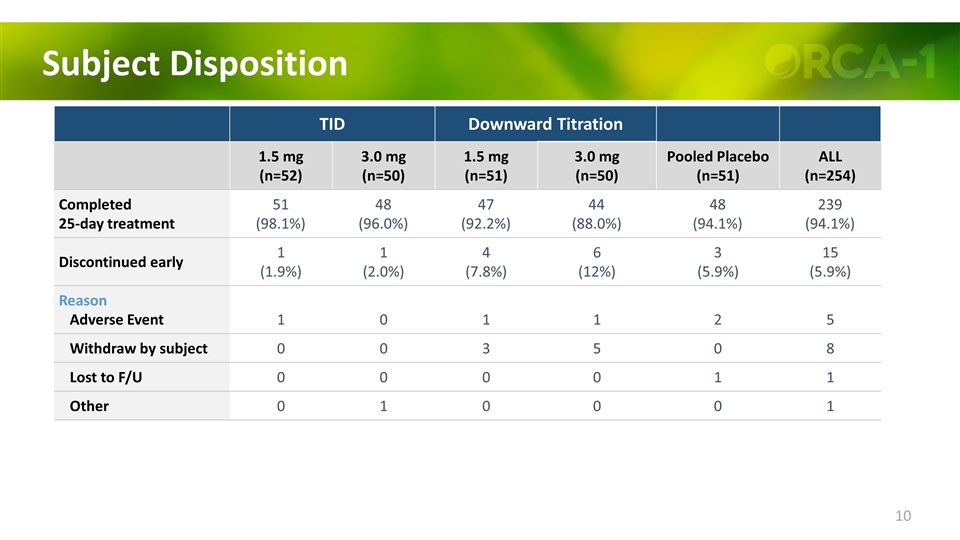
Subject Disposition TID Downward Titration 1.5 mg (n=52) 3.0 mg (n=50) 1.5 mg (n=51) 3.0 mg (n=50) Pooled Placebo (n=51) ALL (n=254) Completed 25-day treatment 51 (98.1%) 48 (96.0%) 47 (92.2%) 44 (88.0%) 48 (94.1%) 239 (94.1%) Discontinued early 1 (1.9%) 1 (2.0%) 4 (7.8%) 6 (12%) 3 (5.9%) 15 (5.9%) Reason Adverse Event 1 0 1 1 2 5 Withdraw by subject 0 0 3 5 0 8 Lost to F/U 0 0 0 0 1 1 Other 0 1 0 0 0 1
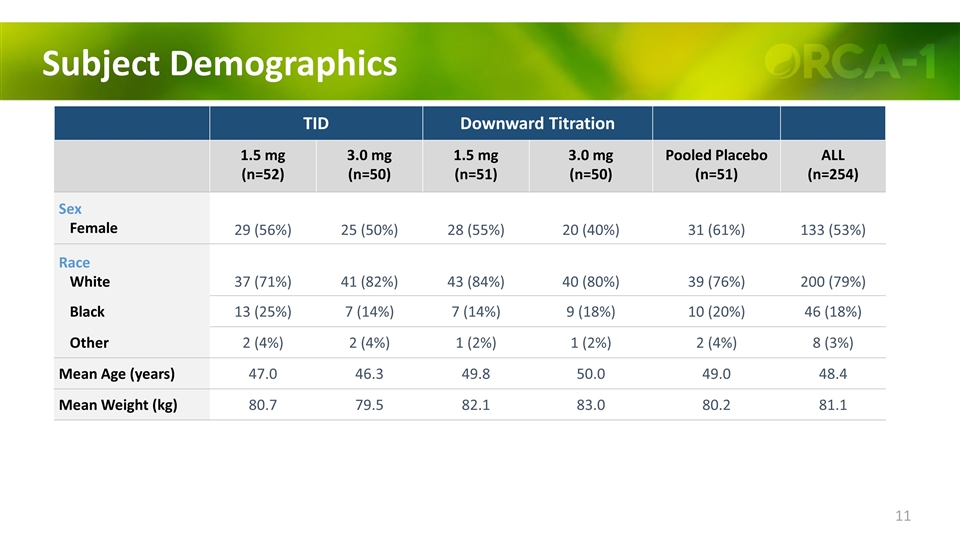
Subject Demographics TID Downward Titration 1.5 mg (n=52) 3.0 mg (n=50) 1.5 mg (n=51) 3.0 mg (n=50) Pooled Placebo (n=51) ALL (n=254) Sex Female 29 (56%) 25 (50%) 28 (55%) 20 (40%) 31 (61%) 133 (53%) Race White 37 (71%) 41 (82%) 43 (84%) 40 (80%) 39 (76%) 200 (79%) Black 13 (25%) 7 (14%) 7 (14%) 9 (18%) 10 (20%) 46 (18%) Other 2 (4%) 2 (4%) 1 (2%) 1 (2%) 2 (4%) 8 (3%) Mean Age (years) 47.0 46.3 49.8 50.0 49.0 48.4 Mean Weight (kg) 80.7 79.5 82.1 83.0 80.2 81.1
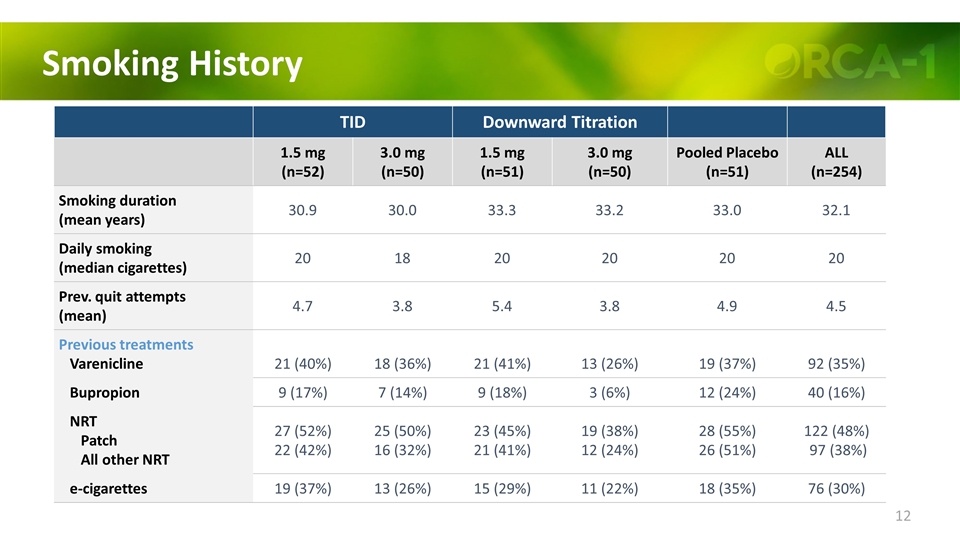
Smoking History TID Downward Titration 1.5 mg (n=52) 3.0 mg (n=50) 1.5 mg (n=51) 3.0 mg (n=50) Pooled Placebo (n=51) ALL (n=254) Smoking duration (mean years) 30.9 30.0 33.3 33.2 33.0 32.1 Daily smoking (median cigarettes) 20 18 20 20 20 20 Prev. quit attempts (mean) 4.7 3.8 5.4 3.8 4.9 4.5 Previous treatments Varenicline 21 (40%) 18 (36%) 21 (41%) 13 (26%) 19 (37%) 92 (35%) Bupropion 9 (17%) 7 (14%) 9 (18%) 3 (6%) 12 (24%) 40 (16%) NRT Patch All other NRT 27 (52%) 22 (42%) 25 (50%) 16 (32%) 23 (45%) 21 (41%) 19 (38%) 12 (24%) 28 (55%) 26 (51%) 122 (48%) 97 (38%) e-cigarettes 19 (37%) 13 (26%) 15 (29%) 11 (22%) 18 (35%) 76 (30%)
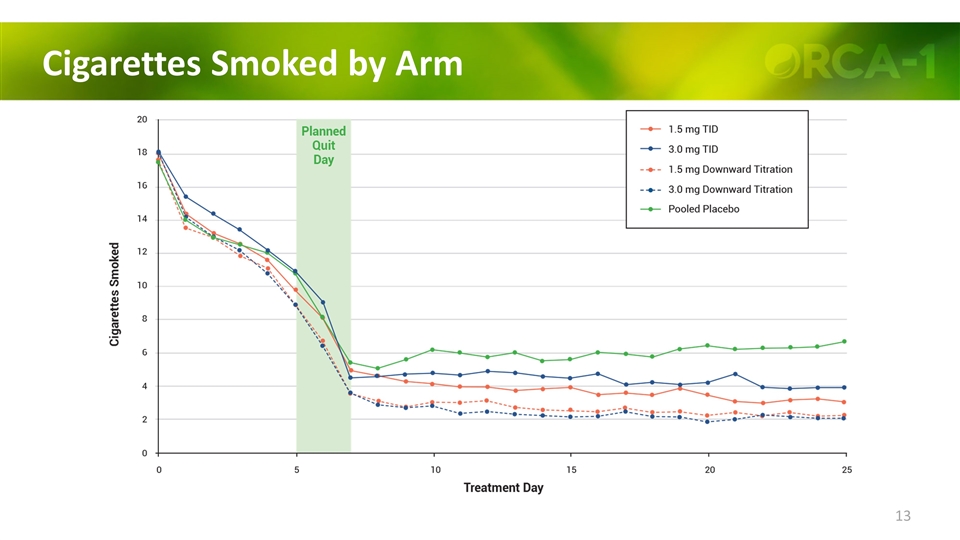
Cigarettes Smoked by Arm
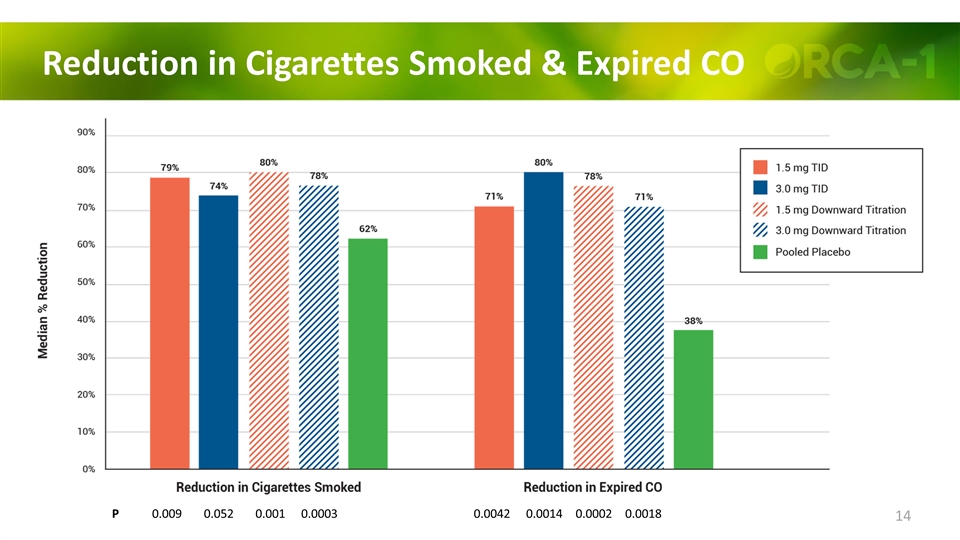
Reduction in Cigarettes Smoked & Expired CO P 0.009 0.052 0.001 0.0003 0.0042 0.0014 0.0002 0.0018
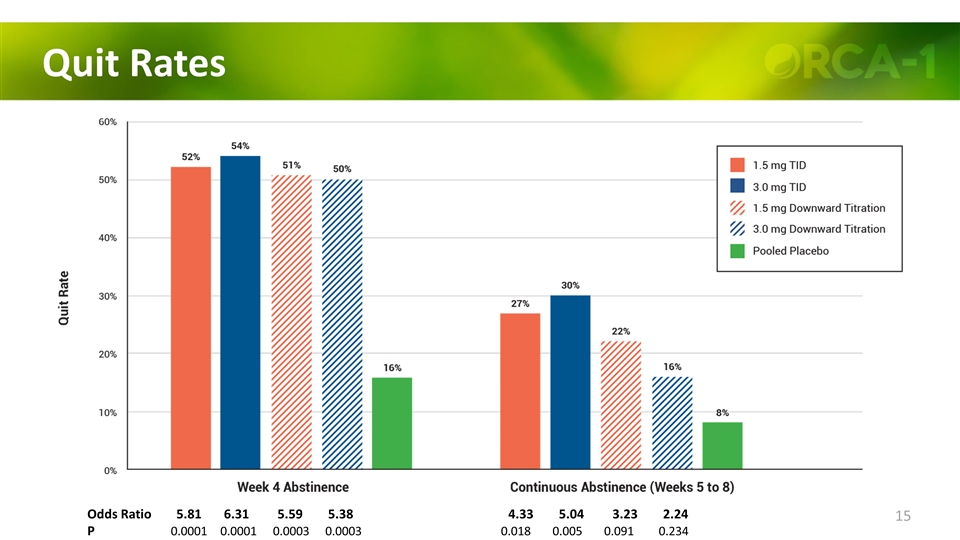
Quit Rates Odds Ratio 5.81 6.31 5.59 5.38 4.33 5.04 3.23 2.24 P 0.0001 0.0001 0.0003 0.0003 0.018 0.005 0.091 0.234
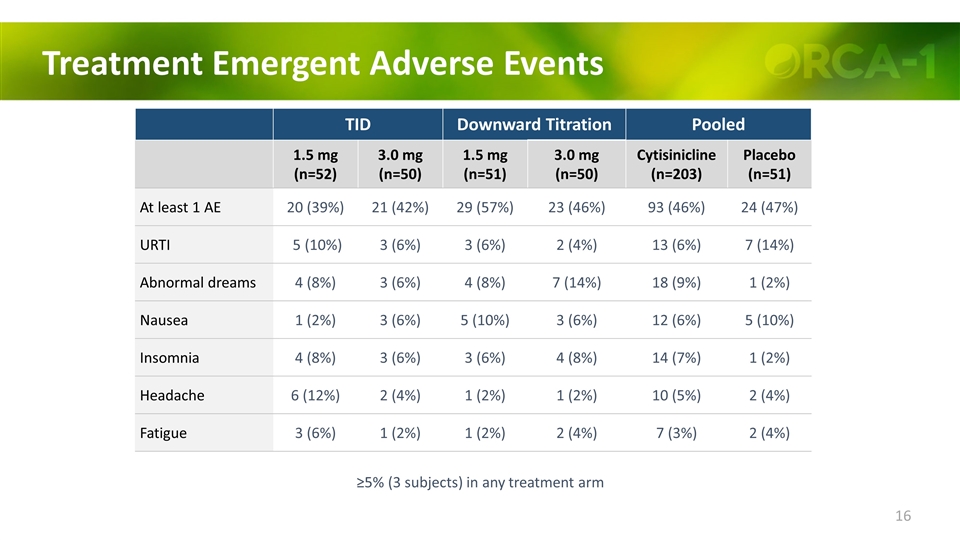
Treatment Emergent Adverse Events TID Downward Titration Pooled 1.5 mg (n=52) 3.0 mg (n=50) 1.5 mg (n=51) 3.0 mg (n=50) Cytisinicline (n=203) Placebo (n=51) At least 1 AE 20 (39%) 21 (42%) 29 (57%) 23 (46%) 93 (46%) 24 (47%) URTI 5 (10%) 3 (6%) 3 (6%) 2 (4%) 13 (6%) 7 (14%) Abnormal dreams 4 (8%) 3 (6%) 4 (8%) 7 (14%) 18 (9%) 1 (2%) Nausea 1 (2%) 3 (6%) 5 (10%) 3 (6%) 12 (6%) 5 (10%) Insomnia 4 (8%) 3 (6%) 3 (6%) 4 (8%) 14 (7%) 1 (2%) Headache 6 (12%) 2 (4%) 1 (2%) 1 (2%) 10 (5%) 2 (4%) Fatigue 3 (6%) 1 (2%) 1 (2%) 2 (4%) 7 (3%) 2 (4%) ≥5% (3 subjects) in any treatment arm

Summary Efficacy Rapid reduction in cigarettes smoked, but not matched with CO in placebo arms Significantly enhanced quit rates Week 4 (end of treatment) 4-week continuous abstinence week 5 to week 8 (off treatment) Safety Low incidence in adverse events - no serious or severe adverse events No clinically-significant changes in vital signs, routine hematology/chemistry, ECG Conclusions Cytisinicline is an effective aid to smoking cessation with an advantageous adverse event profile 3.0 mg TID more efficacious overall with no increase in adverse events

Summary Thank you

Compliance results from a multicenter, double-blind, randomized, placebo-controlled phase 2b trial comparing two treatment schedules for cytisinicline in adult smokers Nides M.1, Rigotti N.2, Benowitz N.3, Cain D.4, Clarke A.4, Jacobs C.4 1 Los Angeles Clinical Trials, Burbank, United States 2 Massachusetts General Hospital/Harvard Medical School, Boston, United States 3 University of California San Francisco, San Francisco, United States 4 Achieve Life Sciences, Inc., Seattle, United States

Outline Study Details & Results Identified Study Challenges Potential Solutions Results Conclusions
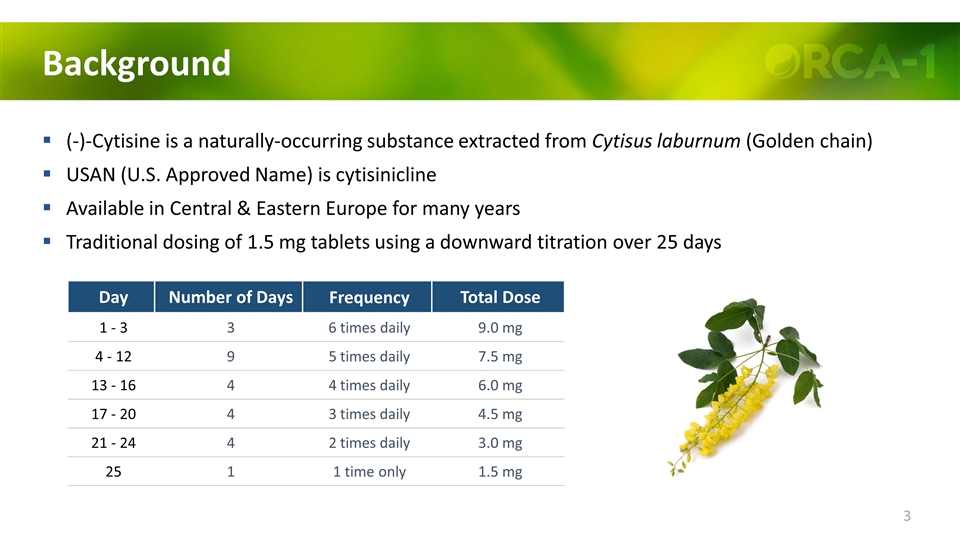
Background (-)-Cytisine is a naturally-occurring substance extracted from Cytisus laburnum (Golden chain) USAN (U.S. Approved Name) is cytisinicline Available in Central & Eastern Europe for many years Traditional dosing of 1.5 mg tablets using a downward titration over 25 days Day Number of Days Frequency Total Dose 1 - 3 3 6 times daily 9.0 mg 4 - 12 9 5 times daily 7.5 mg 13 - 16 4 4 times daily 6.0 mg 17 - 20 4 3 times daily 4.5 mg 21 - 24 4 2 times daily 3.0 mg 25 1 1 time only 1.5 mg
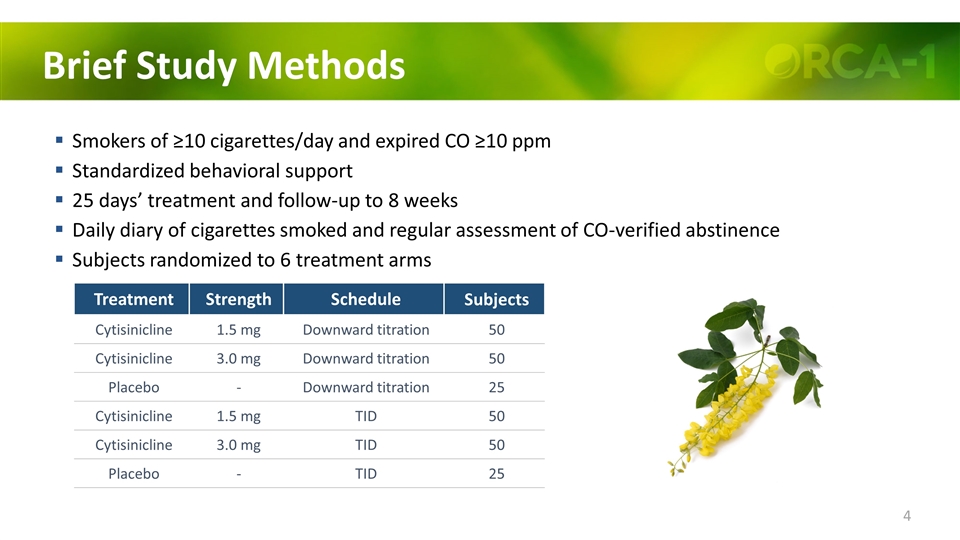
Brief Study Methods Smokers of ≥10 cigarettes/day and expired CO ≥10 ppm Standardized behavioral support 25 days’ treatment and follow-up to 8 weeks Daily diary of cigarettes smoked and regular assessment of CO-verified abstinence Subjects randomized to 6 treatment arms Treatment Strength Schedule Subjects Cytisinicline 1.5 mg Downward titration 50 Cytisinicline 3.0 mg Downward titration 50 Placebo - Downward titration 25 Cytisinicline 1.5 mg TID 50 Cytisinicline 3.0 mg TID 50 Placebo - TID 25
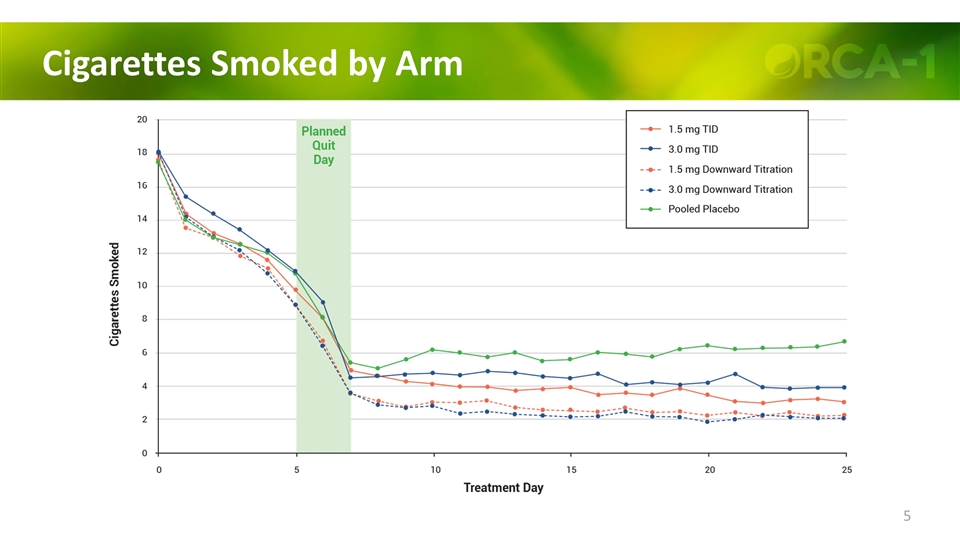
Cigarettes Smoked by Arm
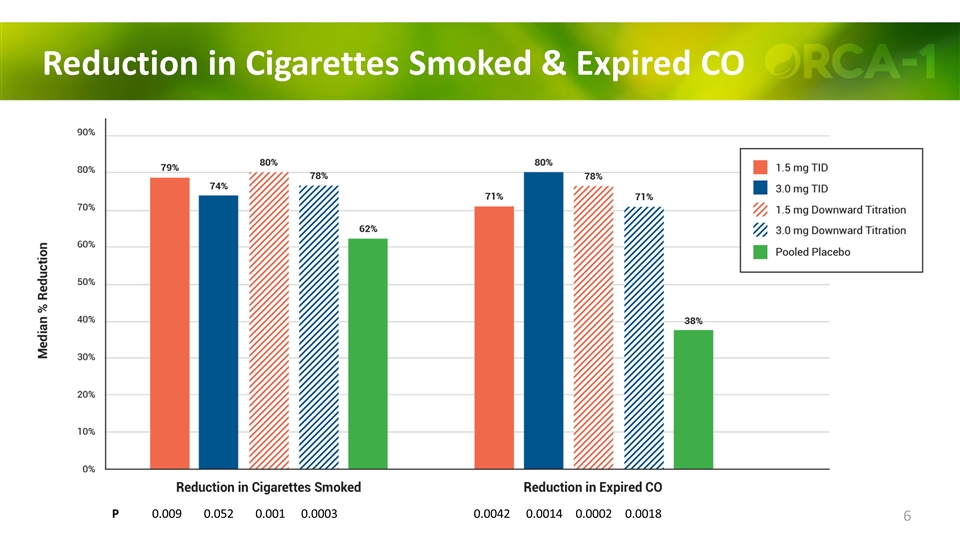
Reduction in Cigarettes Smoked & Expired CO P 0.009 0.052 0.001 0.0003 0.0042 0.0014 0.0002 0.0018
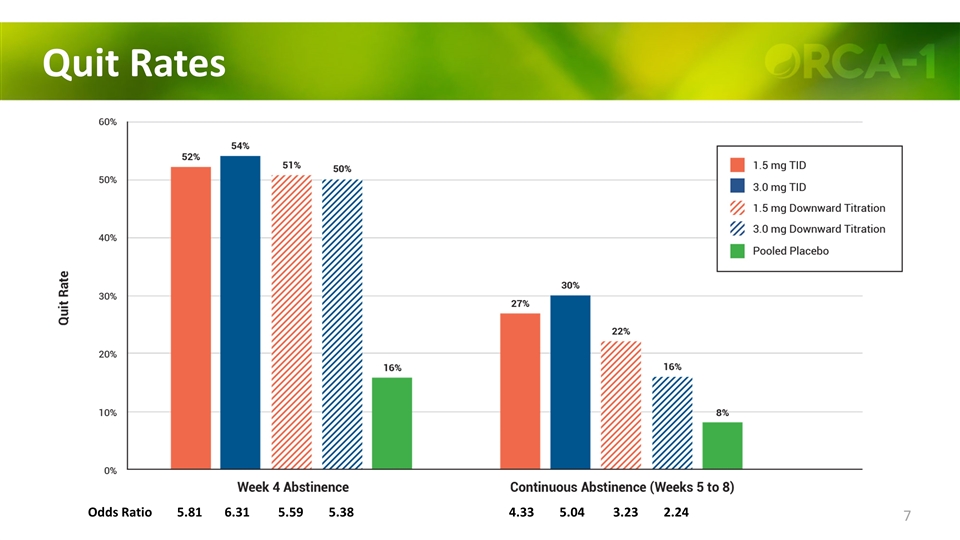
Quit Rates Odds Ratio 5.81 6.31 5.59 5.38 4.33 5.04 3.23 2.24

Summary Efficacy Rapid reduction in cigarettes smoked, but not matched with CO in placebo arms Significantly enhanced quit rates Week 4 (end of treatment) 4-week continuous abstinence week 5 to week 8 (off treatment) Safety Low incidence in adverse events - no serious or severe adverse events No clinically-significant changes in vital signs, routine hematology/chemistry, ECG Conclusions Cytisinicline is an effective aid to smoking cessation with an advantageous adverse event profile 3.0 mg TID more efficacious overall with no increase in adverse events

Study Challenges Very Frequent Visits Complex dosing schedules Required to take 2 tablets at each dosing Multiple doses in a given day impacting work/life schedules Daily dosing that changed over time (downward titration and TID) during the treatment period Self-administered oral medication
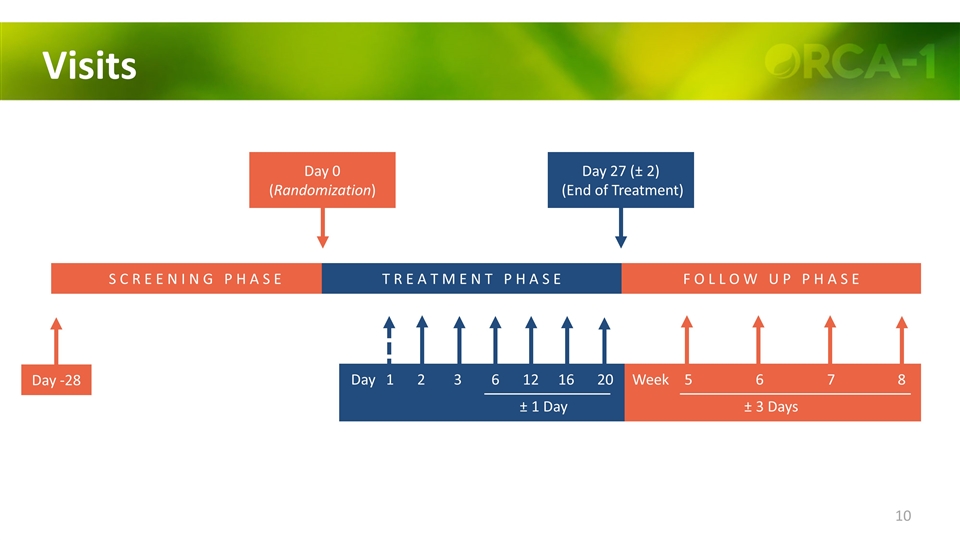
Visits S C R E E N I N G P H A S E F O L L O W U P P H A S E Day -28 Day 0 (Randomization) Day 27 (± 2) (End of Treatment) 1 2 3 6 12 16 Day 5 6 7 8 Week ± 3 Days T R E A T M E N T P H A S E 20 ± 1 Day
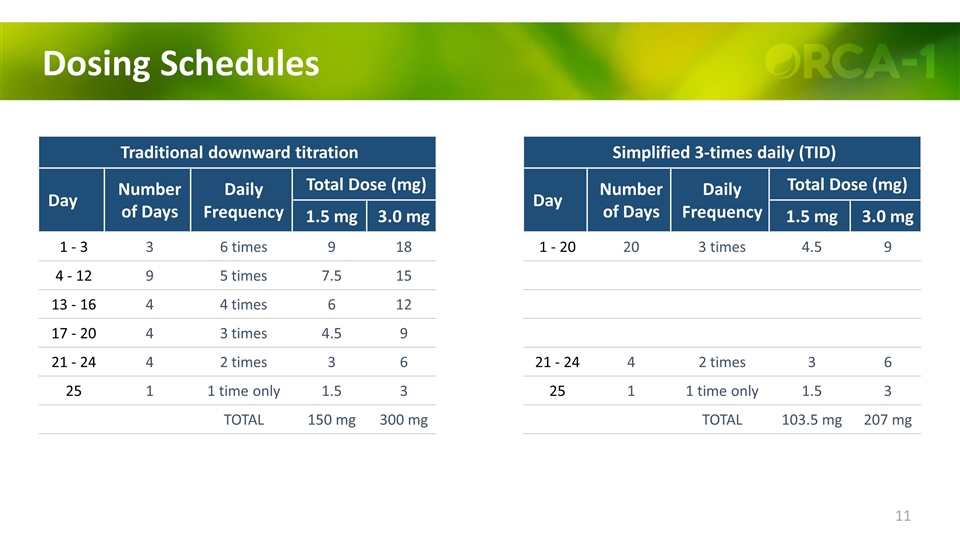
Dosing Schedules Traditional downward titration Day Number of Days Daily Frequency Total Dose (mg) 1.5 mg 3.0 mg 1 - 3 3 6 times 9 18 4 - 12 9 5 times 7.5 15 13 - 16 4 4 times 6 12 17 - 20 4 3 times 4.5 9 21 - 24 4 2 times 3 6 25 1 1 time only 1.5 3 TOTAL 150 mg 300 mg Simplified 3-times daily (TID) Day Number of Days Daily Frequency Total Dose (mg) 1.5 mg 3.0 mg 1 - 20 20 3 times 4.5 9 21 - 24 4 2 times 3 6 25 1 1 time only 1.5 3 TOTAL 103.5 mg 207 mg

Solutions Study subject interaction and training Initial call on first day of treatment Requirement for frequent clinic visits during treatment (7 visits over 27 days & 11 in total) One-on-one review of packaging and dosing requirements with subject provided at randomization Detailed subject reference manual provided Individual Dose Segment Packaging Treatment blister packs designed to coincide with treatment visits Treatment blister packs administered & collected as subject progressed throughout study Separate Drug Accountability Logs maintained by pharmacy and reviewed by study monitors

Solutions Use of text-messaging reminders for each dosing and diary entries Electronic Diary Initial in-depth training provided by the site Baseline “testing” requirement of 7 consecutive days for screening On-study daily entry requirements (date & time for each dose) eDiary Entry Oversight by clinical site (on-line during treatment)

Solutions – Friendly & Detailed Packaging
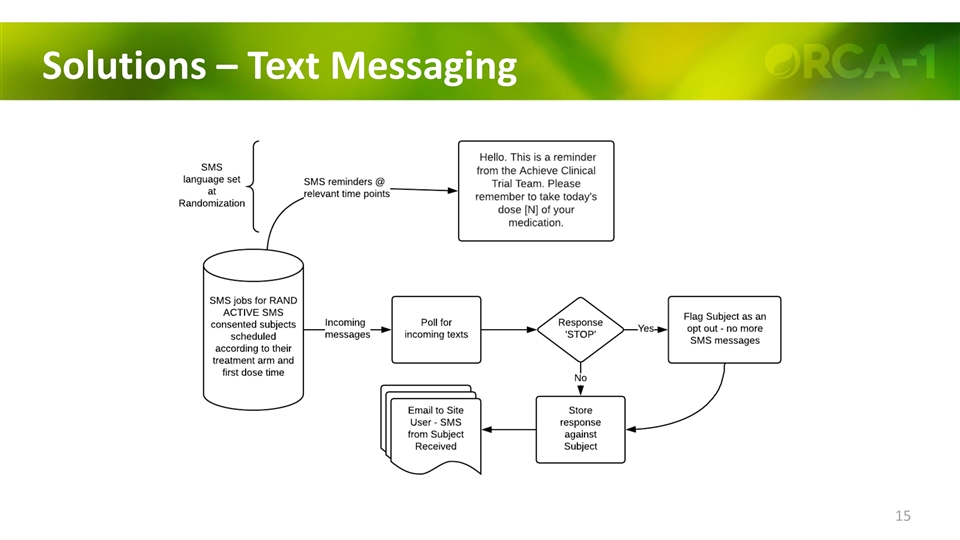
Solutions – Text Messaging

Results – Text Messaging Consent to receive Text Messages on Personal Cell Phones 244 (96%) subjects consented to receive Text Messages 5 (2%) subjects requested to have texts turned off at some point during treatment (4/5 were on downward titration schedule) 20 (8%) subjects had their text turned off by the site at some point during treatment as they discontinued for various reasons Exit questionnaire provided many positive comments ”Extremely helpful and great reminder” “Saved me a couple of times when I forgot” “Kept me on track"
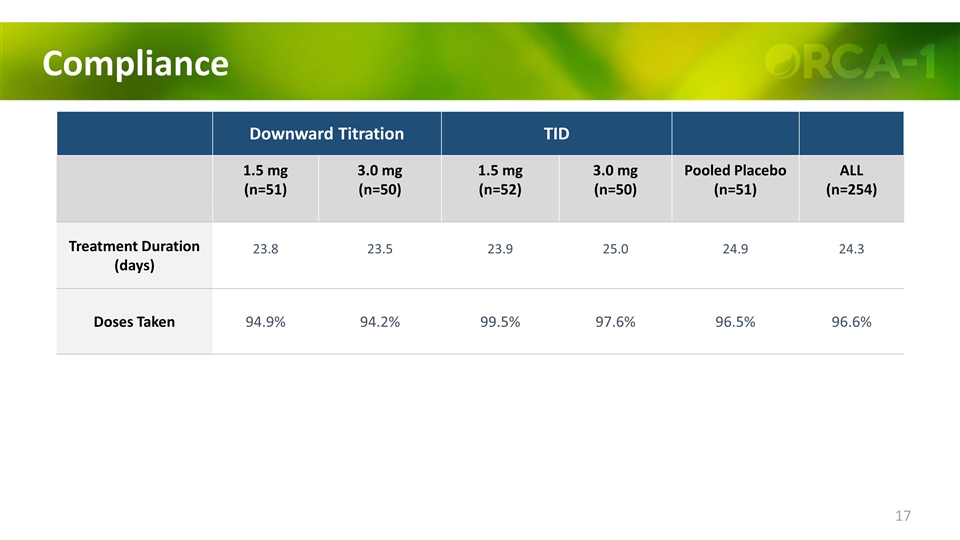
Compliance Downward Titration TID 1.5 mg (n=51) 3.0 mg (n=50) 1.5 mg (n=52) 3.0 mg (n=50) Pooled Placebo (n=51) ALL (n=254) Treatment Duration (days) 23.8 23.5 23.9 25.0 24.9 24.3 Doses Taken 94.9% 94.2% 99.5% 97.6% 96.5% 96.6%
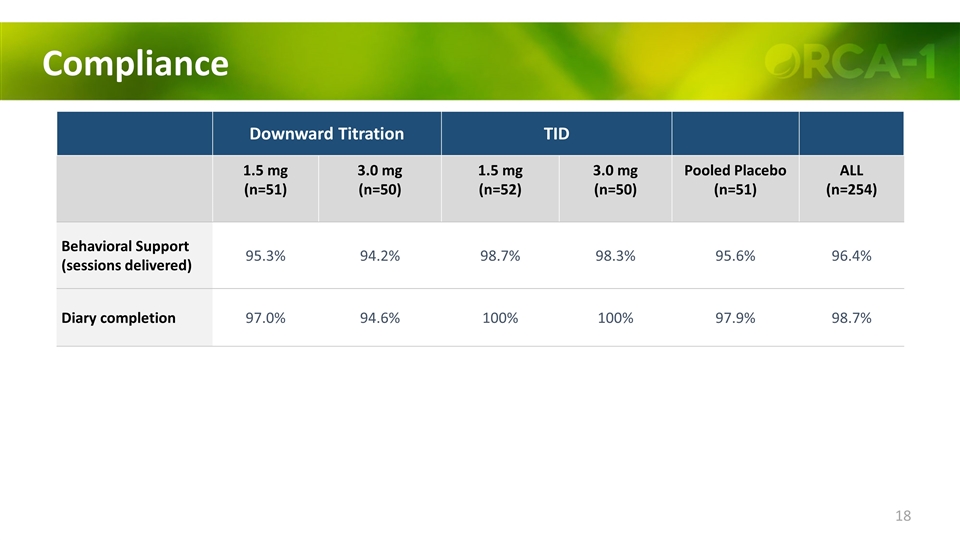
Compliance Downward Titration TID 1.5 mg (n=51) 3.0 mg (n=50) 1.5 mg (n=52) 3.0 mg (n=50) Pooled Placebo (n=51) ALL (n=254) Behavioral Support (sessions delivered) 95.3% 94.2% 98.7% 98.3% 95.6% 96.4% Diary completion 97.0% 94.6% 100% 100% 97.9% 98.7%

Conclusions This was a complex Phase 2b trial Addressing known challenges yielded favorable compliance rates These tools and procedures will be utilized again in the Phase 3 program

Summary Thank you