EX-99.2
Published on February 20, 2018

- Achieve Life Sciences - Committed to advancing cytisine as a smoking cessation aid to address the global tobacco addiction epidemic Exhibit 99.2

Forward Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the timing of planned clinical development activities of cytisine; the projected path toward potential regulatory approval; the safety, efficacy and commercial potential of cytisine; the potential market for cytisine; the benefits of cytisine relative to competitors; the anticipated benefits of cytisine; plans, objectives, expectations and intentions with respect to future operations. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Achieve Life Science, Inc. (Achieve) may not actually achieve its plans or product development goals in a timely manner, if at all, or otherwise carry out the intentions or meet the expectations or projections disclosed in these forward-looking statements. These statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward-looking statements, including, among others, general business and economic conditions; the need for and ability to obtain additional financing; the risk that cytisine may not demonstrate the hypothesized or expected benefits; the risk that cytisine will not receive regulatory approval or be successfully commercialized; the risk that new developments in the smoking cessation landscape require changes in business strategy or clinical development plans; the risk that Achieve's intellectual property may not be adequately protected; other risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates that are safe and effective for use as human therapeutics; and the other factors described in the risk factors set forth in Achieve's filings with the Securities and Exchange Commission from time to time, including the final Proxy Statement/Prospectus/Information Statement filed pursuant to Rule 424(b)(3) in connection with Achieve's recent merger, and Achieve's Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q. Achieve undertakes no obligation to update the forward-looking statements contained herein or to reflect events or circumstances occurring after the date hereof, other than as may be required by applicable law.

Phase I/II Repeat Dose PK/PD Preliminary Study Results February 2018

Cytisine Overview Cytisine is a plant-based alkaloid with high affinity and specificity for neuronal nicotinic (α4β2) receptors. Established smoking cessation treatment that has been available in Central and Eastern Europe since the 1960’s; Estimated 20 million smokers have been treated with cytisine. Two Phase 3 trials were conducted and published in NEJM in 2011 & 2014 demonstrating efficacy and safety of cytisine as an aid to smoking cessation. Achieve Life Sciences, in collaboration with research partners, is pursuing clinical development efforts required for FDA and other regulatory approvals.
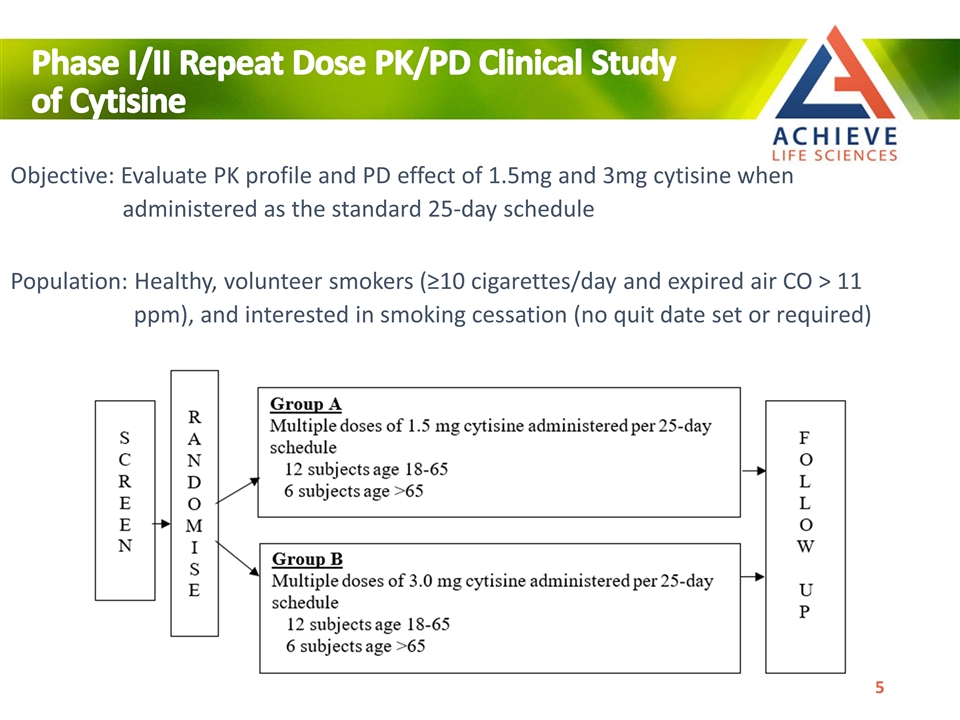
Objective: Evaluate PK profile and PD effect of 1.5mg and 3mg cytisine when administered as the standard 25-day schedule Population: Healthy, volunteer smokers (≥10 cigarettes/day and expired air CO > 11 ppm), and interested in smoking cessation (no quit date set or required) Phase I/II Repeat Dose PK/PD Clinical Study of Cytisine
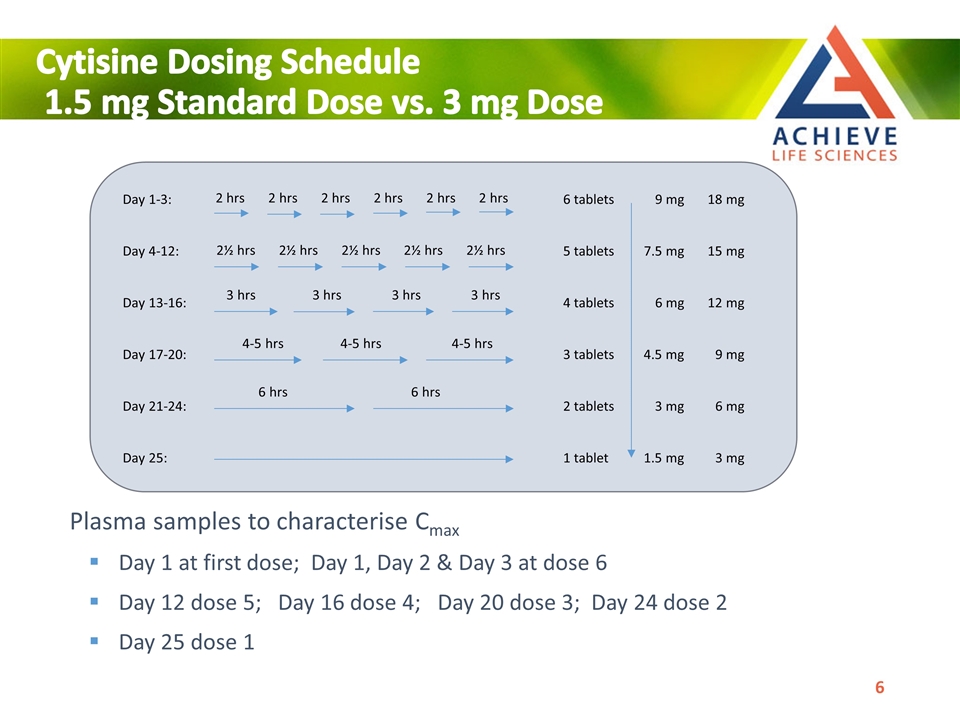
Cytisine Dosing Schedule 1.5 mg Standard Dose vs. 3 mg Dose Plasma samples to characterise Cmax Day 1 at first dose; Day 1, Day 2 & Day 3 at dose 6 Day 12 dose 5; Day 16 dose 4; Day 20 dose 3; Day 24 dose 2 Day 25 dose 1 Day 1-3: 6 tablets 9 mg 18 mg 2 hrs 2 hrs 2 hrs 2 hrs 2 hrs 2 hrs Day 4-12: 2½ hrs 2½ hrs 2½ hrs 2½ hrs 2½ hrs 5 tablets 7.5 mg 15 mg Day 13-16: 4 tablets 6 mg 12 mg 3 hrs 3 hrs 3 hrs 3 hrs Day 17-20: 3 tablets 4.5 mg 9 mg 4-5 hrs 4-5 hrs 4-5 hrs Day 21-24: 2 tablets 3 mg 6 mg 6 hrs 6 hrs Day 25: 1 tablet 1.5 mg 3 mg
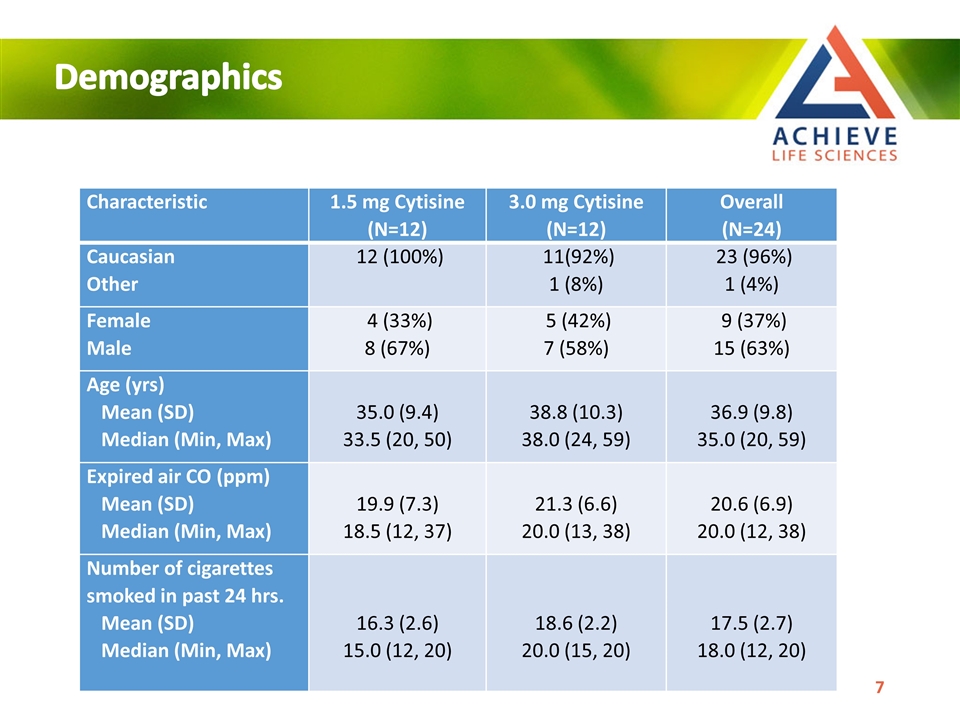
Demographics Characteristic 1.5 mg Cytisine (N=12) 3.0 mg Cytisine (N=12) Overall (N=24) Caucasian Other 12 (100%) 11(92%) 1 (8%) 23 (96%) 1 (4%) Female Male 4 (33%) 8 (67%) 5 (42%) 7 (58%) 9 (37%) 15 (63%) Age (yrs) Mean (SD) Median (Min, Max) 35.0 (9.4) 33.5 (20, 50) 38.8 (10.3) 38.0 (24, 59) 36.9 (9.8) 35.0 (20, 59) Expired air CO (ppm) Mean (SD) Median (Min, Max) 19.9 (7.3) 18.5 (12, 37) 21.3 (6.6) 20.0 (13, 38) 20.6 (6.9) 20.0 (12, 38) Number of cigarettes smoked in past 24 hrs. Mean (SD) Median (Min, Max) 16.3 (2.6) 15.0 (12, 20) 18.6 (2.2) 20.0 (15, 20) 17.5 (2.7) 18.0 (12, 20)
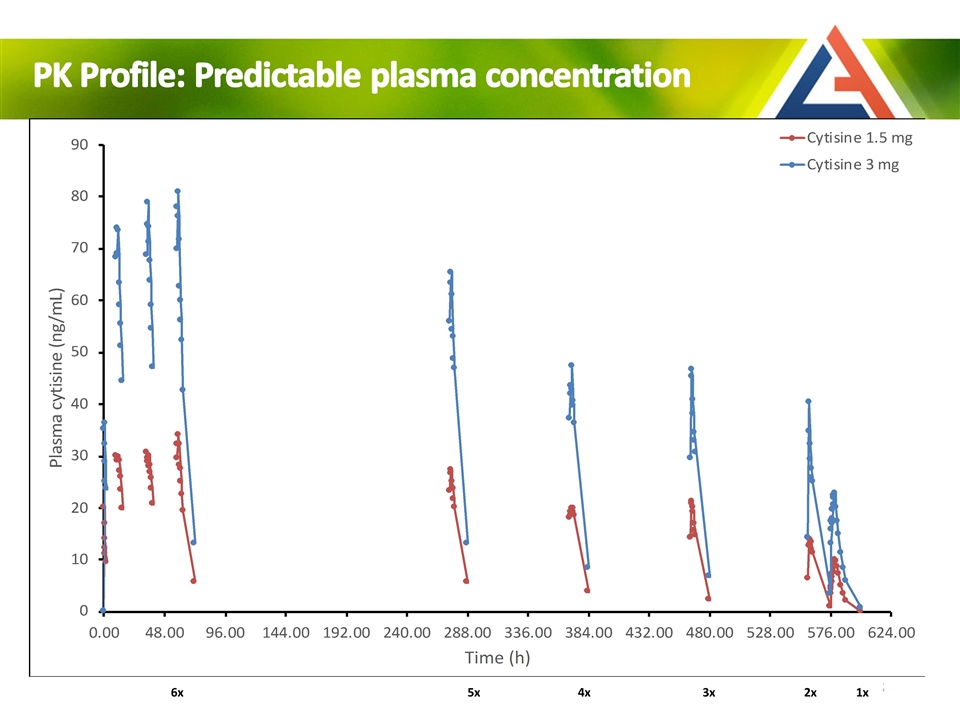
Day 1 Day 2 Day 3 Day 12 Day 16 Day 20 Day 24 Day 25 Day 12 6x 5x 4x 3x 2x 1x PK Profile: Predictable plasma concentration
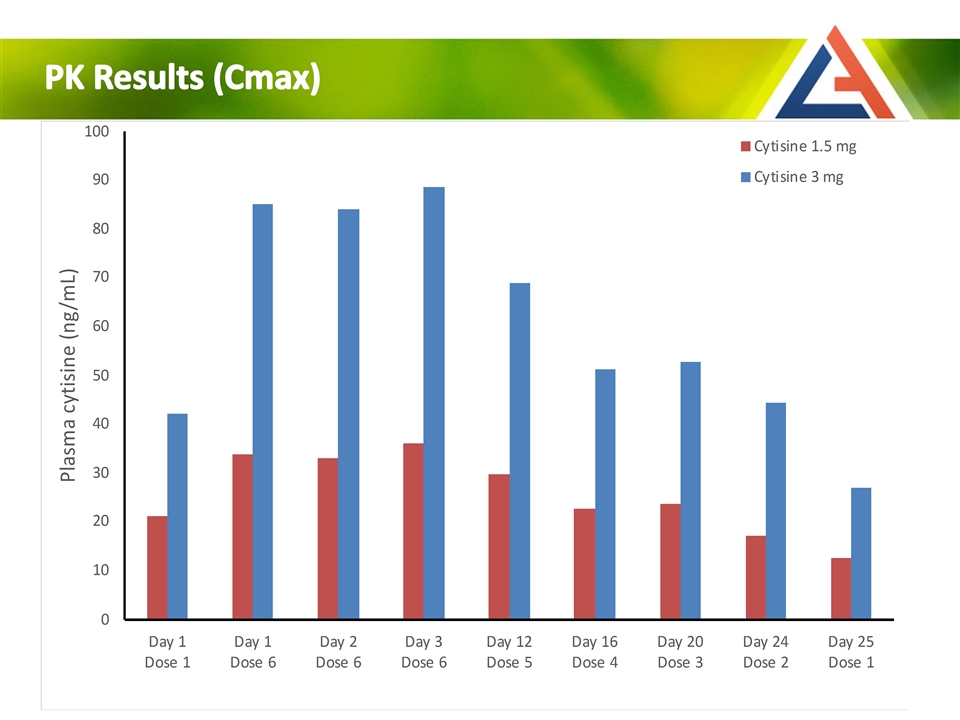
PK Results (Cmax)
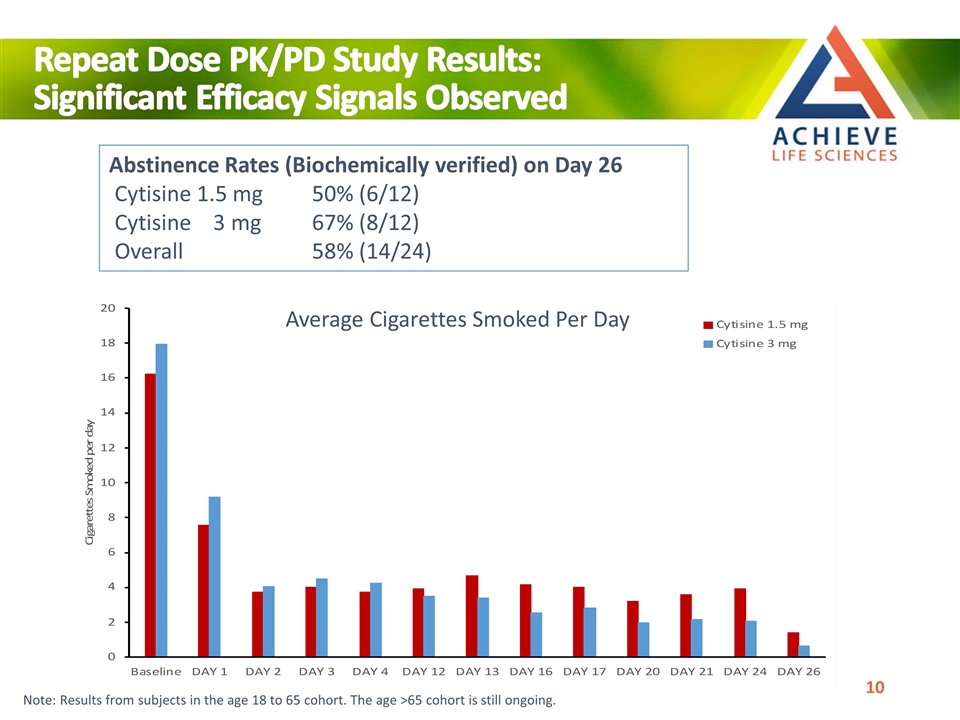
Abstinence Rates (Biochemically verified) on Day 26 Cytisine 1.5 mg 50% (6/12) Cytisine 3 mg 67% (8/12) Overall 58% (14/24) Repeat Dose PK/PD Study Results: Significant Efficacy Signals Observed Average Cigarettes Smoked Per Day Note: Results from subjects in the age 18 to 65 cohort. The age >65 cohort is still ongoing.
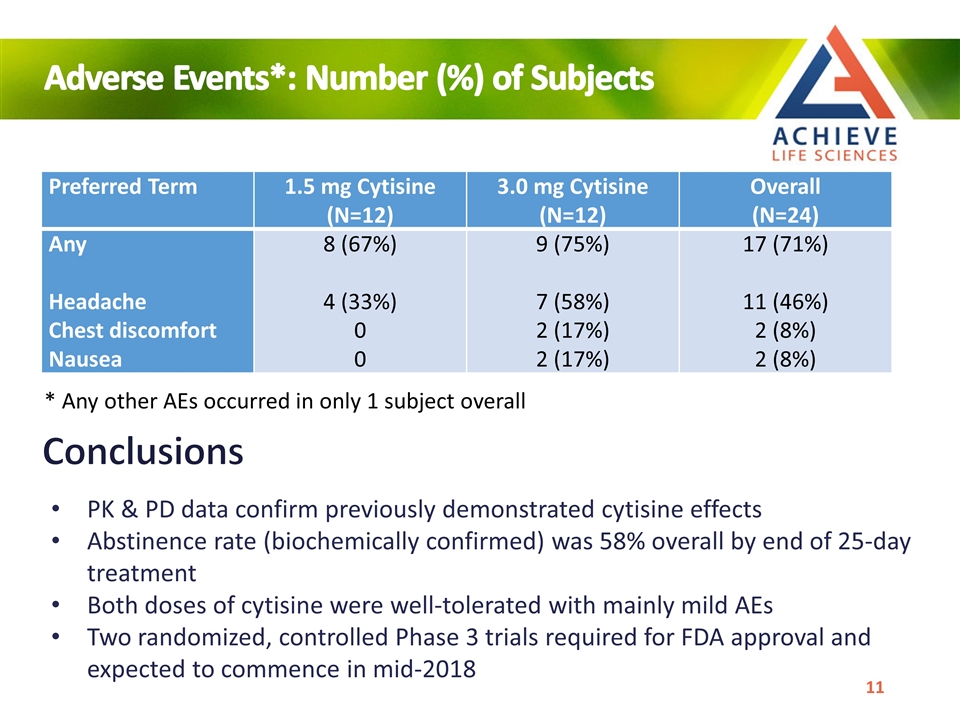
Preferred Term 1.5 mg Cytisine (N=12) 3.0 mg Cytisine (N=12) Overall (N=24) Any Headache Chest discomfort Nausea 8 (67%) 4 (33%) 0 0 9 (75%) 7 (58%) 2 (17%) 2 (17%) 17 (71%) 11 (46%) 2 (8%) 2 (8%) Conclusions PK & PD data confirm previously demonstrated cytisine effects Abstinence rate (biochemically confirmed) was 58% overall by end of 25-day treatment Both doses of cytisine were well-tolerated with mainly mild AEs Two randomized, controlled Phase 3 trials required for FDA approval and expected to commence in mid-2018 Adverse Events*: Number (%) of Subjects * Any other AEs occurred in only 1 subject overall