EX-99.1
Published on January 6, 2017
January 2017 Achieve Life Science, Inc. Exhibit 99.1 |

This
presentation contains forward-looking statements, including, but not limited to, statements regarding the terms, timing, conditions to and anticipated completion of the proposed merger; the expected
ownership of the combined company and the composition of the combined companys
board of directors and management team; the anticipated distribution to
OncoGenex Pharmaceuticals, Inc. (OngoGenex) stockholders of contingent
value rights (CVRs) and the terms, timing and value of such CVRs; the timing of planned clinical development activities of cytisine; the projected path toward potential regulatory approval;
the safety, efficacy and commercial potential of cytisine; plans, objectives,
expectations and intentions with respect to future operations. All
statements other than statements of historical fact are statements that
could be deemed forward-looking statements. Achieve Life Science, Inc. (Achieve)
and/or OncoGenex may not actually achieve the proposed merger, or any
plans or product development goals in a timely manner, if at all, or
otherwise carry out the intentions or meet the expectations or projections disclosed in these forward-looking statements. These statements are based on management's current expectations and
beliefs and are subject to a number of risks, uncertainties and assumptions that could
cause actual results to differ materially from those described in the
forward-looking statements, including, among others, the failure of
the Achieve or OncoGenex stockholders to approve the transaction; the failure of either party to meet the closing conditions of the transaction; delays in completing the transaction and the risk that the
transaction may not be completed at all; the success of the combined businesses;
operating costs and business disruption during the pendency of and
following the proposed merger; general business and economic
conditions; the need for and ability to obtain additional financing; the ability to source sufficient amounts of cytisine to meet commercial demand; and the risks associated with the process of developing,
obtaining regulatory approval for and commercializing drug candidates that are safe and
effective for use as human therapeutics. Achieve undertakes no obligation
to update the forward-looking statements contained herein or to
reflect events or circumstances occurring after the date hereof, other than as may be required by applicable law. Forward Looking Statements 2 |

This
communication is being made in respect of the proposed merger involving OncoGenex Pharmaceuticals, Inc. and Achieve Life Science, Inc. OncoGenex will file with the Securities and Exchange
Commission, or SEC, a current report on Form 8-K, which will include the merger
agreement and related documents. In addition, OncoGenex intends to file a
registration statement on Form S-4 with the SEC, which will contain a
joint proxy statement/prospectus and other relevant materials, and plans to file with the SEC other documents regarding the proposed transaction. The final joint proxy statement/prospectus will
be sent to the stockholders of OncoGenex and Achieve. The joint proxy
statement/prospectus will contain information about OncoGenex, Achieve,
the proposed merger and related matters. STOCKHOLDERS ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS (INCLUDING ANY AMENDMENTS OR SUPPLEMENTS) AND OTHER DOCUMENTS FILED WITH THE SEC CAREFULLY IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE, AS THEY WILL CONTAIN IMPORTANT INFORMATION THAT STOCKHOLDERS SHOULD CONSIDER BEFORE MAKING A DECISION ABOUT THE MERGER AND RELATED MATTERS. In
addition to receiving the joint proxy statement/prospectus and
proxy card by mail, stockholders will also be able to obtain the joint proxy statement/prospectus, as well as other filings containing information about OncoGenex, without charge, from the SECs website
(http://www.sec.gov) or, without charge, by directing a written request
to: OncoGenex Pharmaceuticals,
Inc., 19820 North Creek Parkway,
Suite 201, Bothell, WA 98011, Attention: Investor Relations or to
Achieve Life Science, Inc., 30 Sunnyside Avenue, Mill Valley, CA 94941,
Attention: Rick Stewart. Important Additional Information About
the Merger
3 |

This
communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such
offer, solicitation or sale would be unlawful prior to registration or qualification
under the securities laws of any such jurisdiction. No offering of
securities in connection with the proposed merger shall be made except by
means of a prospectus meeting the requirements of Section 10 of the
Securities Act of 1933, as amended.
Participants in Solicitation
OncoGenex and its executive officers and directors may be deemed to be participants in
the solicitation of proxies from OncoGenexs stockholders with
respect to the matters relating to the proposed merger. Achieve and its
officers and directors may also be deemed a participant in such solicitation. Information regarding OncoGenexs executive officers and directors is available in OncoGenexs proxy statement on
Schedule 14A, filed with the SEC on April 21, 2016. Information regarding any interest
that OncoGenex, Achieve or any of the executive officers or directors of
OncoGenex or Achieve may have in the transaction with Achieve will be set
forth in the joint proxy statement/prospectus that OncoGenex intends to file with the SEC in connection with its stockholder vote on matters relating to the proposed merger. Stockholders will
be able to obtain this information by reading the joint proxy statement/prospectus when
it becomes available.
Important Additional Information About
the Merger 4 |

Achieve
Life Science, Inc. and OncoGenex Pharmaceuticals, Inc. signed definitive
merger agreement January 5, 2017 Upon completion of the merger, Achieve
shareholders are expected to own
75% and OGXI shareholders are expected to
own 25% of the combined company OGXI shareholders are expected to receive contingent value rights for 80% of defined consideration received related to apatorsen Board composition to be 4 designates of Achieve and 3 designates of OGXI Merger expected to be completed in mid-2017 subject to OGXI shareholder approval About the Merger 5 |

Rick
Stewart Chairman & CEO
Chairman & CEO of Ricanto
CEO of Brabant Pharma (acquired by Zogenix) Chairman & CEO of Huxley Pharma (acquired by BioMarin) CEO of Amarin Corp Founder & CBO of SkyePharma (acquired by Vectura) John Bencich, MBA, CPA CFO CFO of OncoGenex CFO of Integrated Diagnostics CFO of Allozyne CFO of Trubion Pharmaceuticals (acquired by Emergent) Cindy Jacobs, PhD, MD CMO CMO of OncoGenex CMO of Corixa (acquired by GSK) VP Clinical Development, CellPro Anthony Clarke, PhD CSO CSO of Ricanto CSO of Brabant Pharma (acquired by Zogenix) CSO of Huxley Pharma (acquired by BioMarin) CSO of Amarin Corp Post-Merger Management Team 6 |

Achieve
is a privately-held, specialty pharma company focused on the
development of cytisine for smoking cessation
In-licensed WW rights to cytisine from Sopharma (excluding Central and Eastern Europe) Cytisine is approved in Central and Eastern Europe for smoking cessation and sold through Sopharma In-market exposure estimated in over 20 million patients An extensive EMA-compliant PSUR/clinical safety database of 8.5 million patients Safety and efficacy are further supported by two Phase 3 clinical studies in over 2,000 patients published in NEJM Overview: Achieve Life Science, Inc. 7 |

Complete FDA-required IND enabling non-clinical studies in
1H 2017 File IND in 2H 2017 Initiate FDA-required Phase 1 Fed/Fasted and Repeat Dose PK studies in 2H 2017 to support future NDA filing Initiate Phase 3 trial in 1H 2018 File MAA in Europe potentially in 1H 2018 Meetings with German, Dutch and Swedish regulators in 1H 2017 Seeking agreement on MAA package and timing Expected Near-Term Milestones 8 |

Nicotine
addiction/smoking cessation = single most important public health issue globally Market Opportunity 9 Smoking cessation is one of the most important public health issues globally Smoking is the #1 cause of preventable deaths worldwide Tobacco consumption is responsible for cancers, cardiovascular, pulmonary and GI diseases More than 480,000 U.S. deaths annually from smoking-related disease Smoking-related healthcare costs in the U.S. are $170 billion annually Medicaid expenditures attributable to smoking-related diseases total nearly $22 billion annually, representing 11% of all expenditures Global smoking cessation market expected to reach $4.4 billion by 2023 44+ million smokers in U.S. alone ( 15% of the U.S. population) According to the Centers for Disease Control and Prevention (CDC), nearly 70% of current smokers have expressed a desire to quit, 40% attempted to quit in the past year, but only 6.2% succeeded |
Cytisine is a plant-based alkyloid found in members of the leguminosae family Partial agonist that binds with high affinity to the 4 2 nicotinic acetylcholine receptor 4 2 nicotinic receptor is well-characterized in addiction, interrupts nicotine craving Well Characterized MOA Two Phase III trials; 2,050 patients, both published in NEJM 10,000 patients in clinical trials to-date Excellent safety profile 8.5 million patients in European PSUR Safety database Strong Ex-U.S. Clinical and Safety Profile IND enabling studies nearing completion IND expected to be filed in 2H 2017 Phase I fed/fasted & repeat dose PK study Phase 3 trial expected to be initiated in 1H 2018 Defined U.S. Regulatory Pathway Oral product approved for smoking cessation in Central and Eastern Europe 20 million patients treated Cost-effective Demonstrated superiority to nicotine replacement in large, randomized trial Similar efficacy to varenicline, with potentially less side effects Market Experience and Product Opportunity Cytisine: Product Overview 10 |

Clinical Overview 11 |
25-day cytisine dosing
regimen or matched placebo Cytisine vs Placebo 6 & 12-month quit rates confirmed by exhaled carbon monoxide levels Primary Endpoint Double-blind, randomized, placebo-controlled; minimal behavioral support
Conducted in Poland with funding support from Medical Research Council,
Cancer Research UK, Wellcome
Foundation, University College London and
others Cytisine 3.4 times more likely to result in smoking cessation after 12 months (p=0.001) Higher than previous studies have shown for varenicline (2.3) and NRT (1.3) Overall adverse events between cytisine and placebo were similar with higher GI events in cytisine group No Serious Adverse Events related to therapy N=740 Heavy smokers Aged 18 or over; Randomized 1:1 Phase 3 Trial TASC (Cytisine vs. Placebo) N Engl J Med; 365:13 Sept 29, 2011 12 |
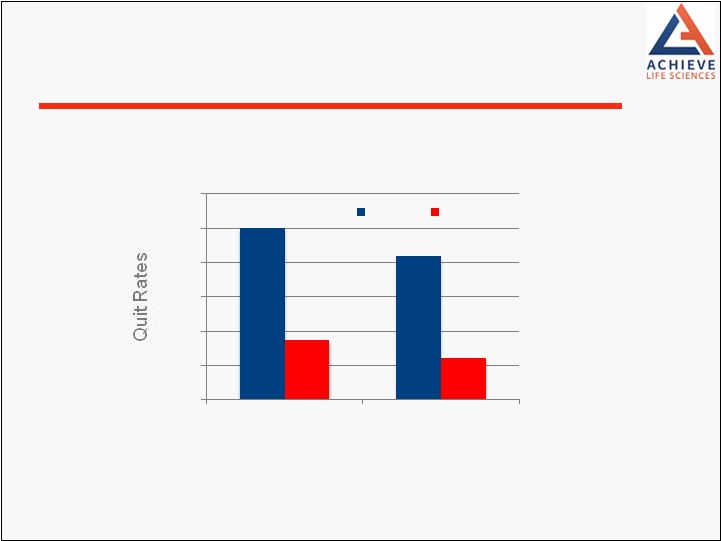
0.0% 2.0% 4.0% 6.0% 8.0% 10.0% 12.0% 6 months 12 months Cytisine Placebo TASC Phase 3 Trial Results N Engl J Med; 365:13 Sept 29, 2011 13 |
25-day cytisine dosing
regimen or 8-week NRT
(patch and/or gum or
lozenge) Cytisine vs. NRT 1, 2, 6-month quit rates Primary Endpoint Randomized, open-label, active-controlled, non-inferiority study design with
moderate behavioral support
Cytisine compared to nicotine replacement therapy (NRT) Conducted by University of Auckland and funded by Health Research Council, New Zealand Cytisine 1.43 times more likely than NRT to result in smoking cessation after 6 months (p=0.002) Not only non-inferior to NRT, but deemed to be superior by investigators No overall difference in adverse effects between cytisine and NRT No Serious Adverse Effects related to therapy N=1,310 Heavy smokers Aged 18 or over; Randomized 1:1 Phase 3 Trial CASCAID (Cytisine vs. NRT) N Engl J Med; 371:25 Dec 18, 2014 14 |
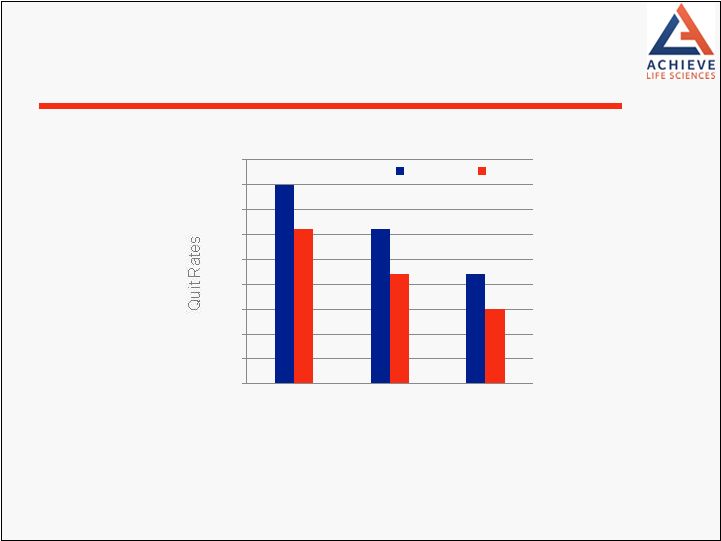
0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 1 Month 2 Months 6 Months Cytisine NRT CASCAID Phase 3 Trial Results N Engl J Med; 371:25 Dec 18, 2014 15 |

Key
findings
No apparent difference in efficacy between cytisine and varenicline
Cytisine has an Relative Risk (RR) = 3.98; varenicline = 2.24 Cytisine trials were conducted with minimal behavioral support, varenicline trials with behavioral support
Behavioral support can increase patient response by 10%-15% Minimal behavioral support may have limited cytisine headline cessation rates Cytisine Pooled RR based on 2 published studies* n=937 RR (CI 95% ) at longest follow-up 3.98 (2.01- 7.87) Varenicline Pooled RR based on 27 published studies N=12,625 RR (CI 95 %) longest follow-up 2.24 (2.06- 2.43) Cochrane Database Review: Cytisine Efficacy Profile Cahill K et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. 16 |
Efficacy
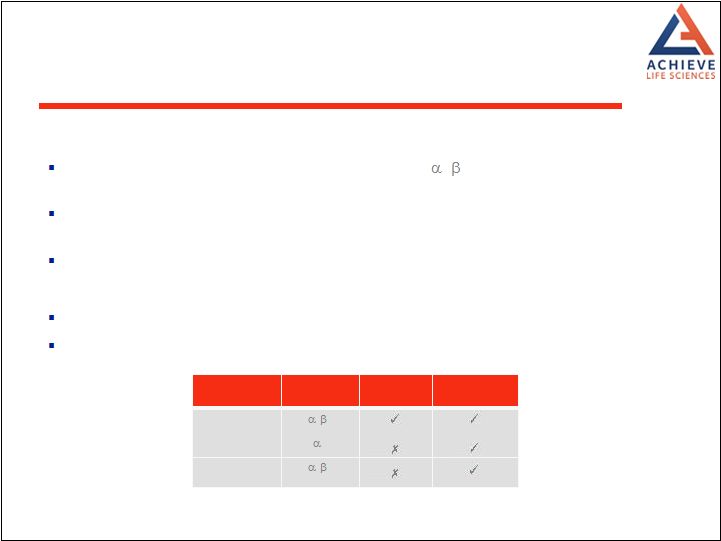
Cytisine and varenicline are partial agonists at the
4 2 nicotinic acetylcholine receptor Activity at this receptor in the ventral tegmental area of the brain mediates therapeutic effect The two drugs have similar efficacy Safety Varenicline interacts potently with a broader range of nicotinic receptors than cytisine Action at these additional receptors may be associated with additional side effects
Site Receptor Cytisine Varenicline Brain 4 2 7 Periphery 3 4 MOA Differences between Cytisine and Varenicline 17 |

Clinical Development
18 |

FDA
directed U.S. regulatory pathway (meeting June 19, 2015) File IND
utilizing non-clinical data package (compliant with current
guidelines) Conduct fed/fasted and repeat dose PK studies Conduct Phase III study in the U.S. Meet with EMA to agree on EU regulatory pathway Meet with national regulators in Germany, Holland & Sweden Determine whether existing MAA dossier is sufficient for MAA submission Include new non-clinical data Include data from TASC and CASCAID Phase 3 trials Potential to file an MAA with EMA with additional datasets Anticipated Path to U.S. and EU Regulatory Approval 19 |
N=36
(to be confirmed) 2
arms fasted, high fat meal Treatment period = single dose End points Primary: Non-inferiority of food versus fasted Phase 1 Planned Phase 1 Fed/Fasted Study 20 |
N=12
(individuals from fed/fasted study) Conducted with fed/fasted study as a
Part A/Part B design Standard dose cytisine, high dose cytisine vs.
placebo Treatment period = 25 days
End points Full PK profile on first and final dose Peak/trough PK profiles after each change (decrease) in dose Phase 1 Planned Phase 1 Repeat-dose Pharmacokinetic Study 21 |
N=2,100 3 arms standard dose cytisine, high dose cytisine vs. placebo Treatment period = 25 days Powered at 90% End points Primary: Biochemically verified abstinence rate at 6-months Secondary: Abstinence rate at 1 & 3-months Patients will be provided behavioral support to U.S. standards Phase 3 Planned Phase 3 Trial Design 22 |

API
& solid dosage form manufacturer
Exclusive supply agreement for 100% of the cytisine
that is required by Achieve
Supply FDA enabling clinical trials Production sufficient to meet global market EU-GMP compliant pharmaceutical manufacturing Opened November 2013 Capacity 4 billion tablets Sopharma Manufacturing & Supply Chain 23 |

Regulatory exclusivity
U.S.: 5 years under Hatch-Waxman
EU: 10 years under Article 8
Exclusive Worldwide Supply of Cytisine
from Sopharma (Bulgaria) Derived from seeds of the tree Golden chain (Cytisus laburnum)
Trees are currently only grown in orchards in Bulgaria controlled by
Sopharma Able to stockpile seeds for future demand 5-7 years required for trees to mature Currently not able to be chemically synthesized Multiple Layers of Product Exclusivity 24 |
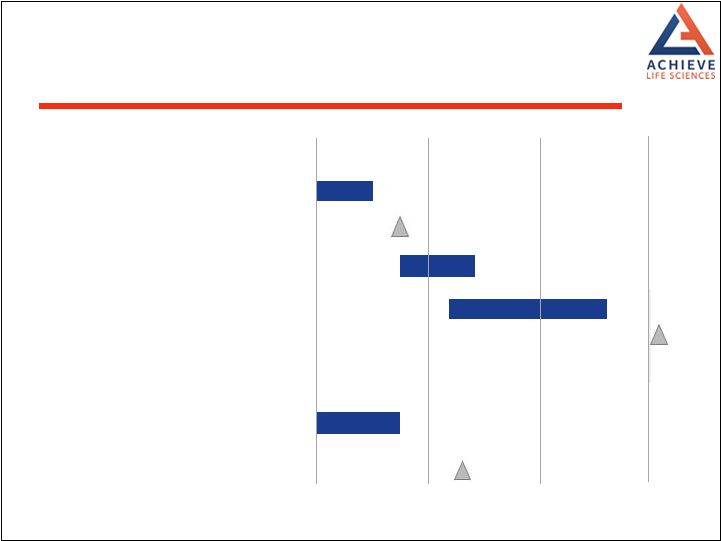
2017 2018 2019 United States Non-clinical studies to support IND File IND with FDA Initiate Phase 1 studies: Fed/Fasted and repeat dose PK studies Initiate Phase 3 File NDA EU Meetings with European regulatory agencies Potential MAA filing Anticipated Path to Commercialization in U.S. and EU 25 |

26 |