EX-99.2
Published on May 23, 2023

ORCA-3 Phase 3 Topline Data NASDAQ: ACHV | May 23, 2023 Exhibit 99.2

Forward Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the timing of planned clinical development activities of cytisinicline; the projected path toward potential regulatory approval; the safety, efficacy and commercial potential of cytisinicline; the potential market for cytisinicline; the benefits of cytisinicline relative to competitors; the anticipated benefits of cytisinicline; plans, objectives, expectations and intentions with respect to future operations. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Achieve Life Sciences, Inc. (“we,” “us,” “our,” or “the Company”) may not actually achieve its plans or product development goals in a timely manner, if at all, or otherwise carry out the intentions or meet the expectations or projections disclosed in these forward-looking statements. These statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward-looking statements, including, among others, general business and economic conditions, including inflation, rising interest rates, instability in the global banking sector and risks related to the COVID-19 pandemic or similar public health crisis; risks related to the Russian military action in Ukraine; the need for and ability to obtain additional financing; the risk that cytisinicline may not demonstrate the hypothesized or expected benefits; the risk that cytisinicline will not receive regulatory approval or be successfully commercialized; the risk that new developments in the smoking cessation landscape require changes in business strategy or clinical development plans; the risk that the Company's intellectual property may not be adequately protected; other risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates that are safe and effective for use as human therapeutics; and the other factors described in the risk factors set forth in the Company's filings with the Securities and Exchange Commission from time to time, including its Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q. The Company undertakes no obligation to update the forward-looking statements contained herein or to reflect events or circumstances occurring after the date hereof, other than as may be required by applicable law.
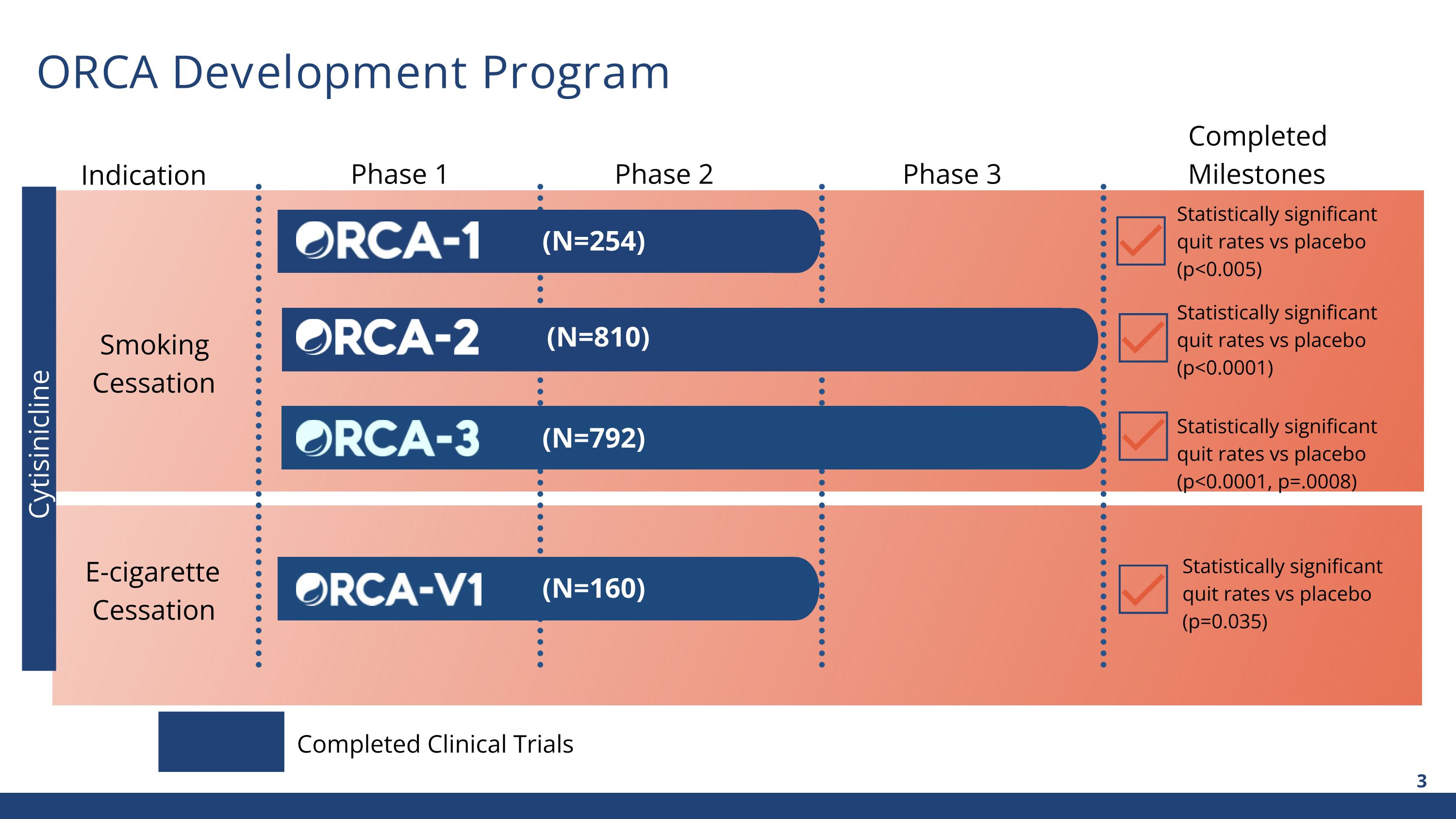
Cytisinicline ORCA Development Program Indication Phase 1 Phase 2 Phase 3 Completed Milestones Smoking Cessation E-cigarette Cessation Completed Clinical Trials Statistically significant quit rates vs placebo (p<0.005) Statistically significant quit rates vs placebo (p<0.0001) Statistically significant quit rates vs placebo (p=0.035) (N=810) (N=254) (N=792) (N=160) Statistically significant quit rates vs placebo (p<0.0001, p=.0008)
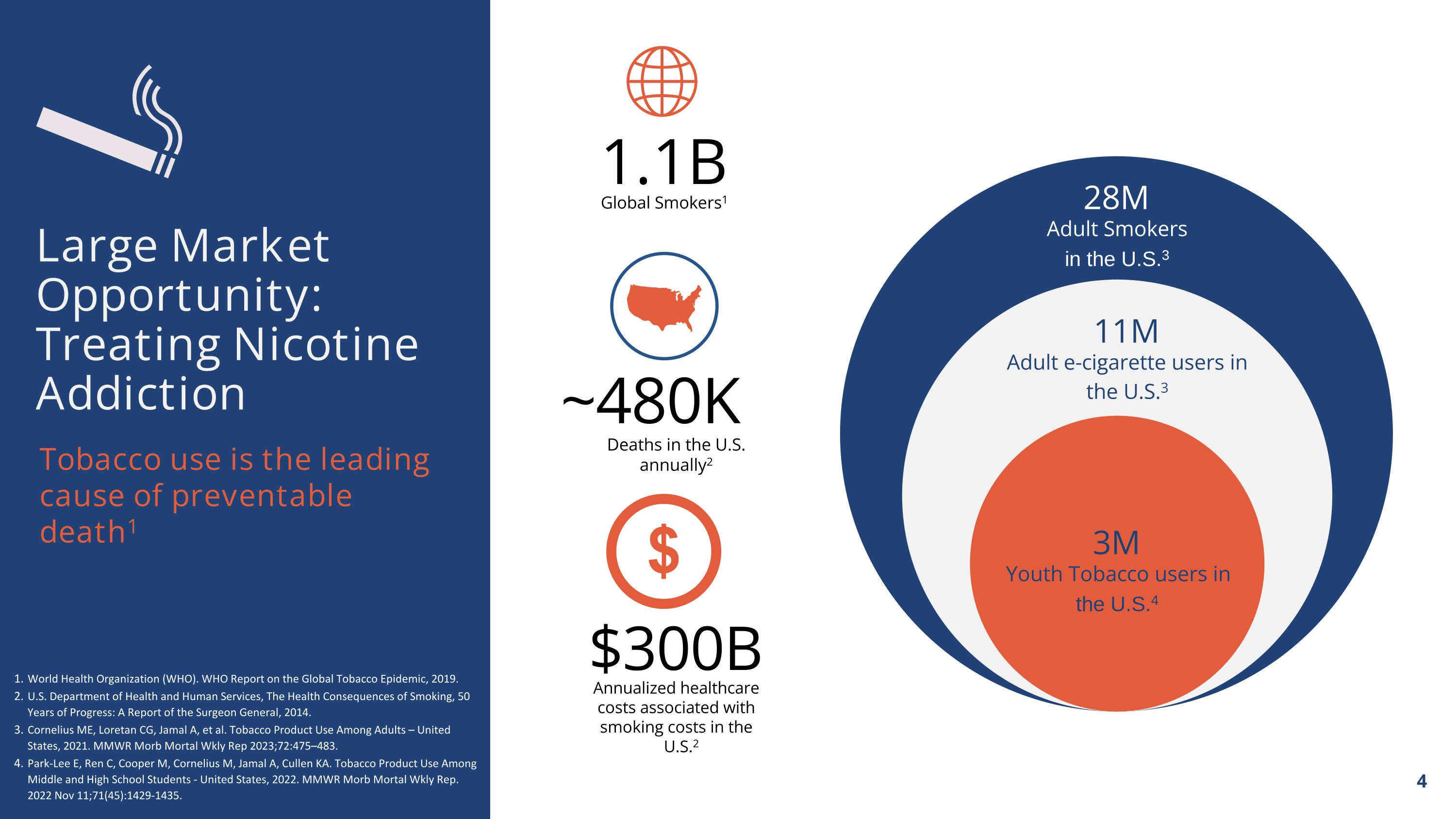
Annualized healthcare costs associated with smoking costs in the U.S.2 $300B 1.1B Global Smokers1 ~480K Deaths in the U.S. annually2 Tobacco use is the leading cause of preventable death1 Large Market Opportunity: Treating Nicotine Addiction 28M Adult Smokers in the U.S.3 11M Adult e-cigarette users in the U.S.3 3M Youth Tobacco users in the U.S.4 World Health Organization (WHO). WHO Report on the Global Tobacco Epidemic, 2019. U.S. Department of Health and Human Services, The Health Consequences of Smoking, 50 Years of Progress: A Report of the Surgeon General, 2014. Cornelius ME, Loretan CG, Jamal A, et al. Tobacco Product Use Among Adults – United States, 2021. MMWR Morb Mortal Wkly Rep 2023;72:475–483. Park-Lee E, Ren C, Cooper M, Cornelius M, Jamal A, Cullen KA. Tobacco Product Use Among Middle and High School Students - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022 Nov 11;71(45):1429-1435.

Forward Looking Statements ORCA-3: Phase 3 Topline Results May 2023
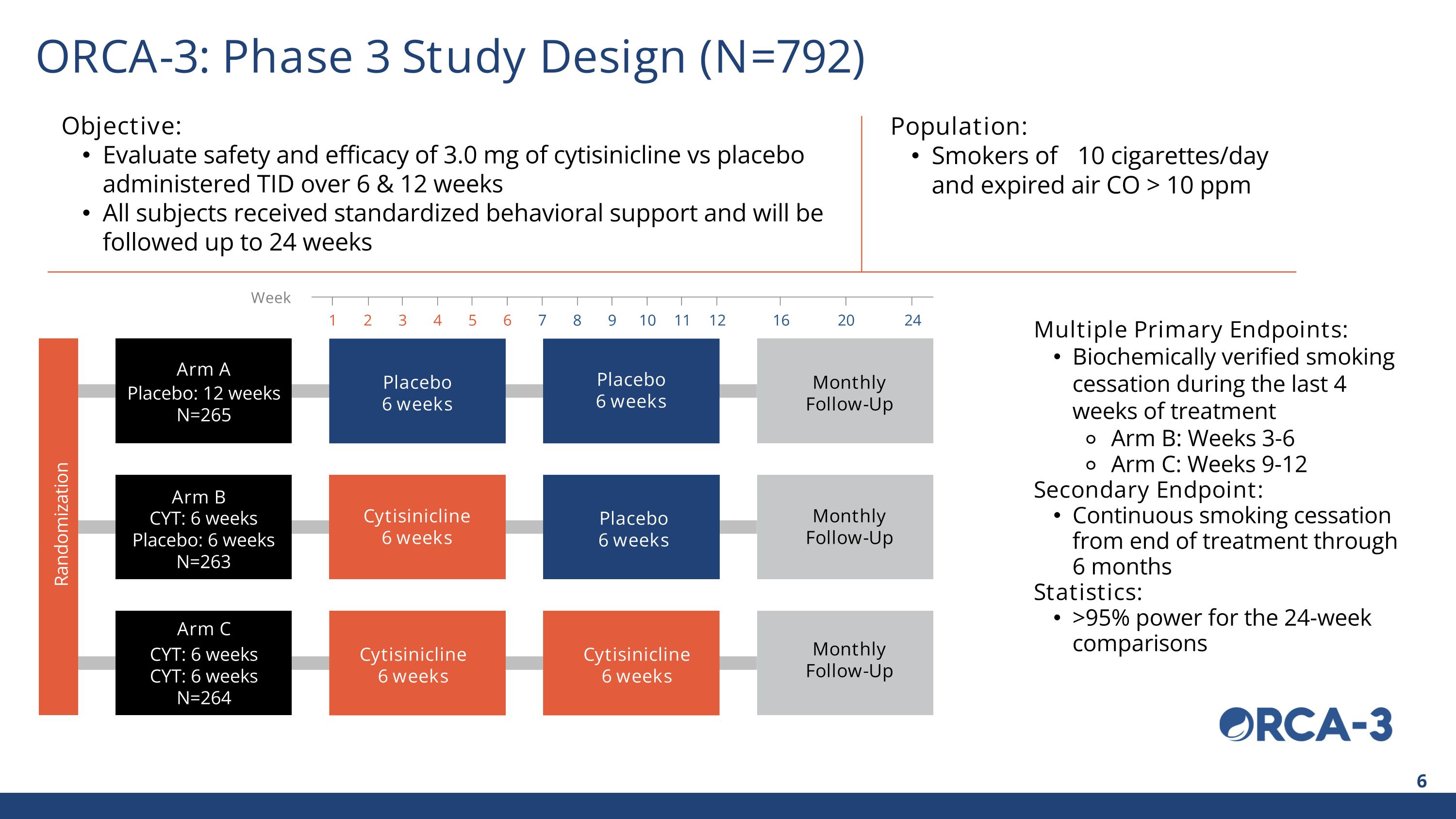
Arm C Arm B Arm A Placebo 6 weeks Cytisinicline 6 weeks Cytisinicline 6 weeks Placebo 6 weeks Placebo 6 weeks Cytisinicline 6 weeks Monthly Follow-Up CYT: 6 weeks CYT: 6 weeks N=264 CYT: 6 weeks Placebo: 6 weeks N=263 Placebo: 12 weeks N=265 Objective: Evaluate safety and efficacy of 3.0 mg of cytisinicline vs placebo administered TID over 6 & 12 weeks All subjects received standardized behavioral support and will be followed up to 24 weeks Population: Smokers of ≥10 cigarettes/day and expired air CO > 10 ppm Multiple Primary Endpoints: Biochemically verified smoking cessation during the last 4 weeks of treatment Arm B: Weeks 3-6 Arm C: Weeks 9-12 Secondary Endpoint: Continuous smoking cessation from end of treatment through 6 months Statistics: >95% power for the 24-week comparisons Week 1 2 3 4 5 6 7 8 9 10 11 12 16 20 24 Randomization ORCA-3: Phase 3 Study Design (N=792) Monthly Follow-Up Monthly Follow-Up
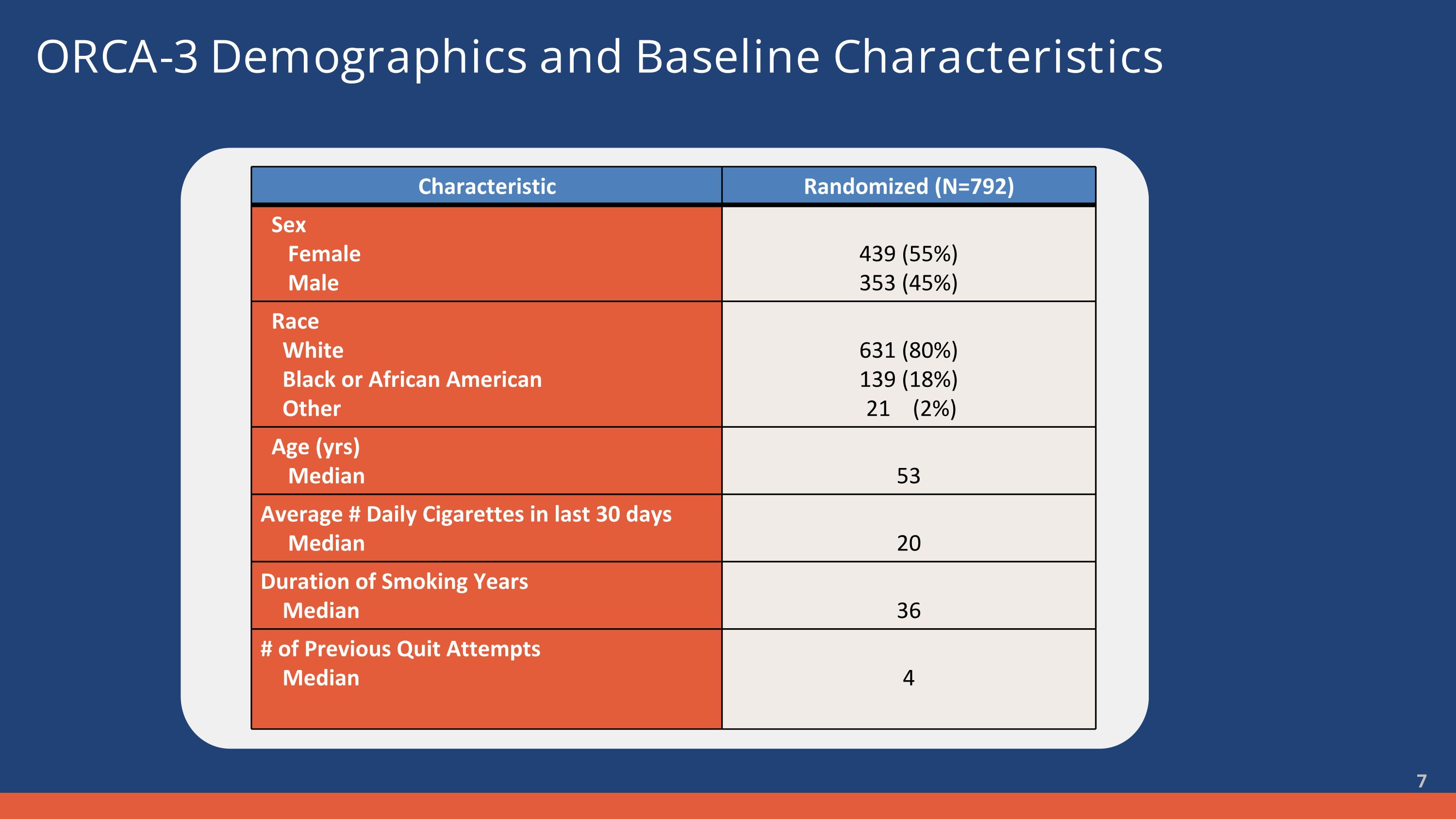
ORCA-3 Demographics and Baseline Characteristics Characteristic Randomized (N=792) Sex Female Male 439 (55%) 353 (45%) Race White Black or African American Other 631 (80%) 139 (18%) 21 (2%) Age (yrs) Median 53 Average # Daily Cigarettes in last 30 days Median 20 Duration of Smoking Years Median 36 # of Previous Quit Attempts Median 4
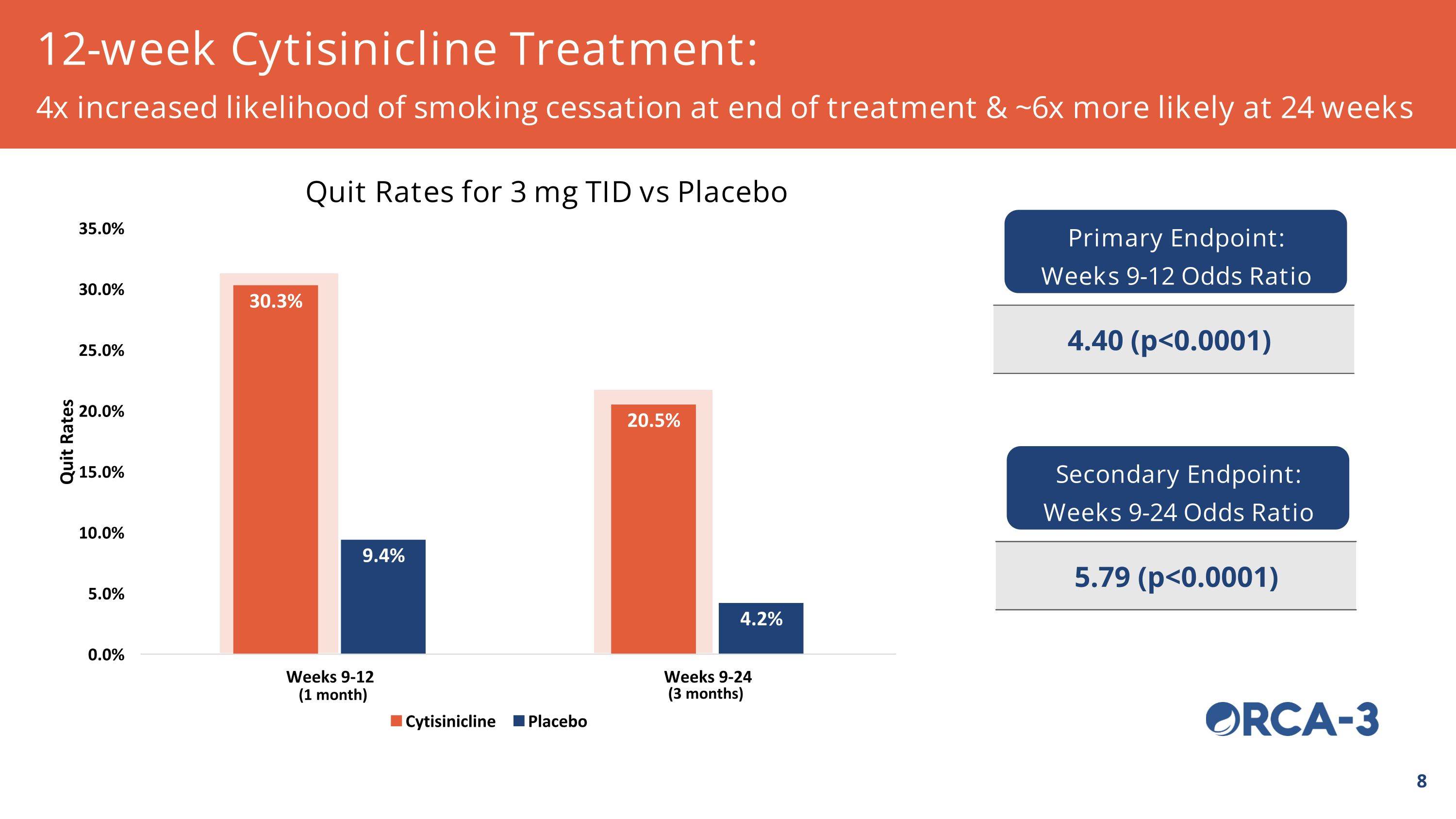
12-week Cytisinicline Treatment: 4x increased likelihood of smoking cessation at end of treatment & ~6x more likely at 24 weeks Quit Rates for 3 mg TID vs Placebo Primary Endpoint: Weeks 9-12 Odds Ratio 4.40 (p<0.0001) Secondary Endpoint: Weeks 9-24 Odds Ratio 5.79 (p<0.0001) (3 months) (1 month)
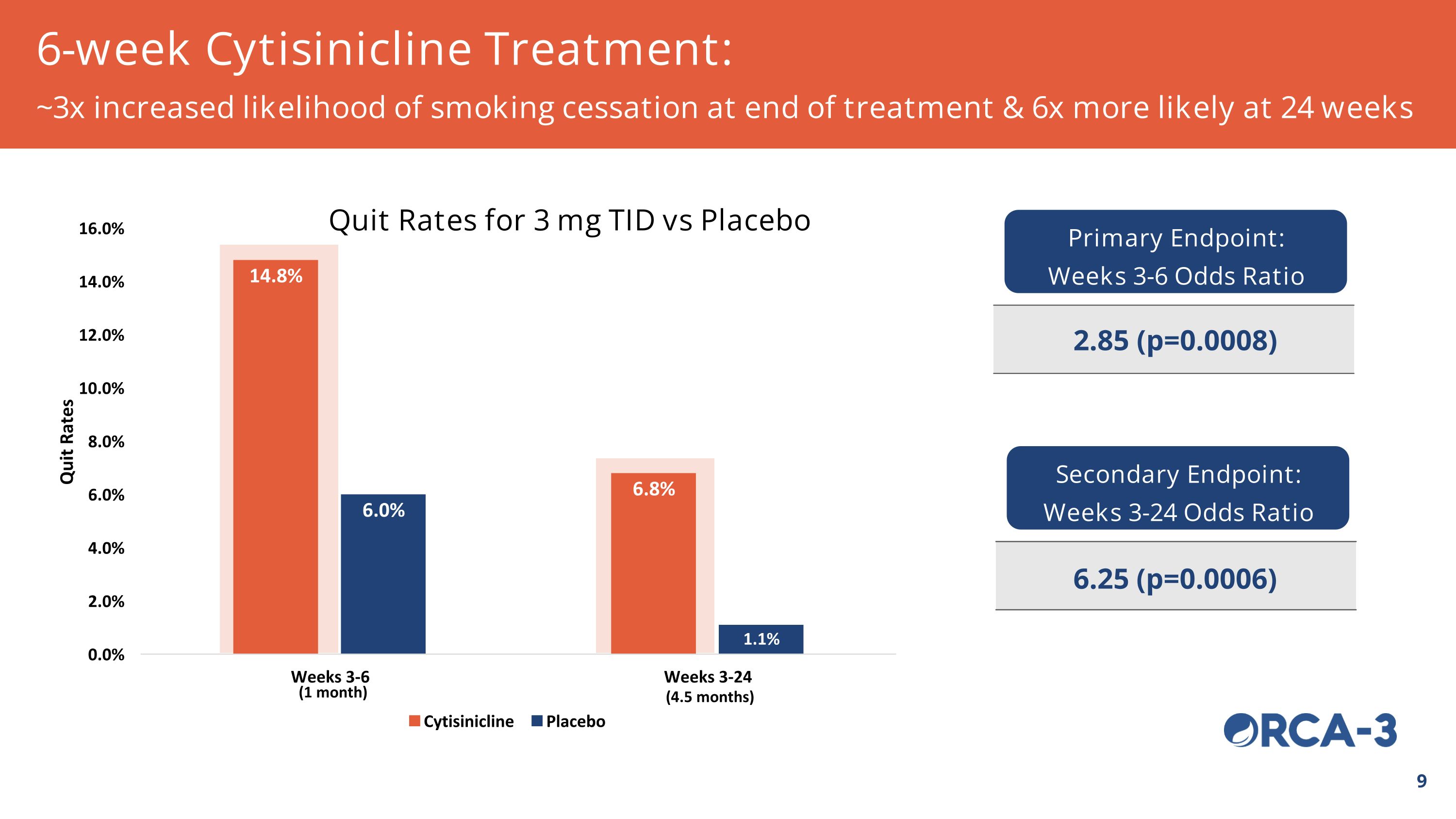
(4.5 months) (1 month) 6-week Cytisinicline Treatment: ~3x increased likelihood of smoking cessation at end of treatment & 6x more likely at 24 weeks Quit Rates for 3 mg TID vs Placebo Primary Endpoint: Weeks 3-6 Odds Ratio 2.85 (p=0.0008) Secondary Endpoint: Weeks 3-24 Odds Ratio 6.25 (p=0.0006)
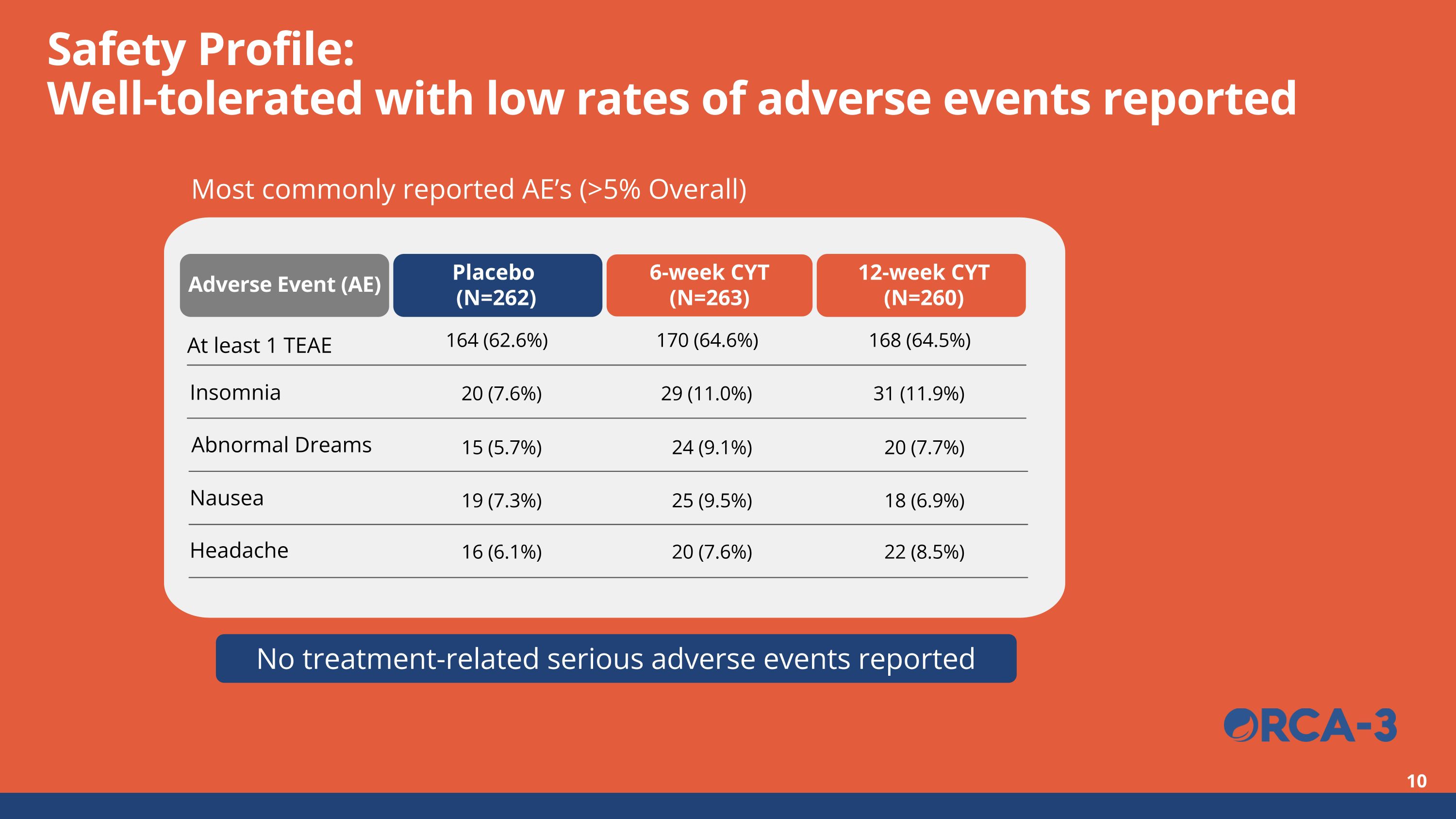
No treatment-related serious adverse events reported Safety Profile:Well-tolerated with low rates of adverse events reported Most commonly reported AE’s (>5% Overall) 164 (62.6%) 170 (64.6%) 168 (64.5%) 20 (7.6%) 29 (11.0%) 31 (11.9%) Adverse Event (AE) Placebo (N=262) 6-week CYT (N=263) 12-week CYT (N=260) At least 1 TEAE Insomnia 15 (5.7%) 24 (9.1%) 20 (7.7%) 19 (7.3%) 25 (9.5%) 18 (6.9%) 16 (6.1%) 20 (7.6%) 22 (8.5%) Headache Nausea Abnormal Dreams
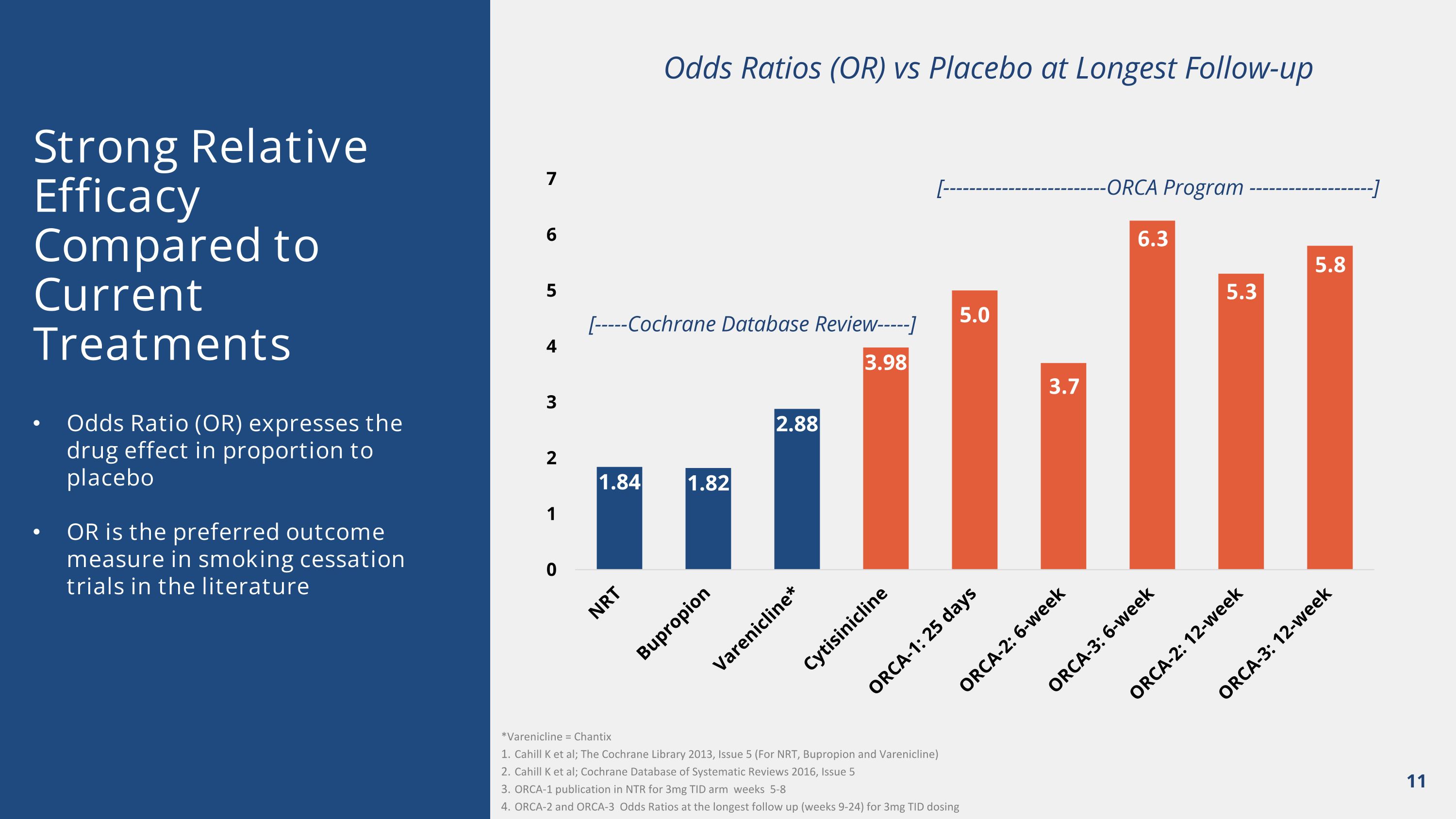
3.7 Strong Relative Efficacy Compared to Current Treatments Odds Ratio (OR) expresses the drug effect in proportion to placebo OR is the preferred outcome measure in smoking cessation trials in the literature [-----Cochrane Database Review-----] *Varenicline = Chantix Cahill K et al; The Cochrane Library 2013, Issue 5 (For NRT, Bupropion and Varenicline) Cahill K et al; Cochrane Database of Systematic Reviews 2016, Issue 5 ORCA-1 publication in NTR for 3mg TID arm weeks 5-8 ORCA-2 and ORCA-3 Odds Ratios at the longest follow up (weeks 9-24) for 3mg TID dosing 5.8 5.3 Odds Ratios (OR) vs Placebo at Longest Follow-up [-------------------------ORCA Program -------------------] 6.3

Cytisinicline is Well Positioned... To Meet the Cessation Needs of Smokers and Patients Battling Nicotine Addiction Dual-acting, differentiated, and highly selective MOA provides minimal adverse events (AEs) and excellent compliance rates Safe & Well-Tolerated Efficacious Robust efficacy demonstrated in multiple, large, randomized controlled trials Short Course of Treatment 6-week treatment with option to extend to 12 weeks for extended benefit Positive In-Market Experience Positive patient & HCP perceptions with no product history of black-box warnings or suicidality Naturally Derived Therapy Sourced from plant-based material which is appealing to specific patient populations Clear Path to Market Collaborative engagement with FDA on NDA-required activities and Affordable Care Act mandates coverage of approved smoking cessation products

Forward Looking Statements APPENDIX

Forward Looking Statements ORCA-2: Phase 3 Study Results April 2022
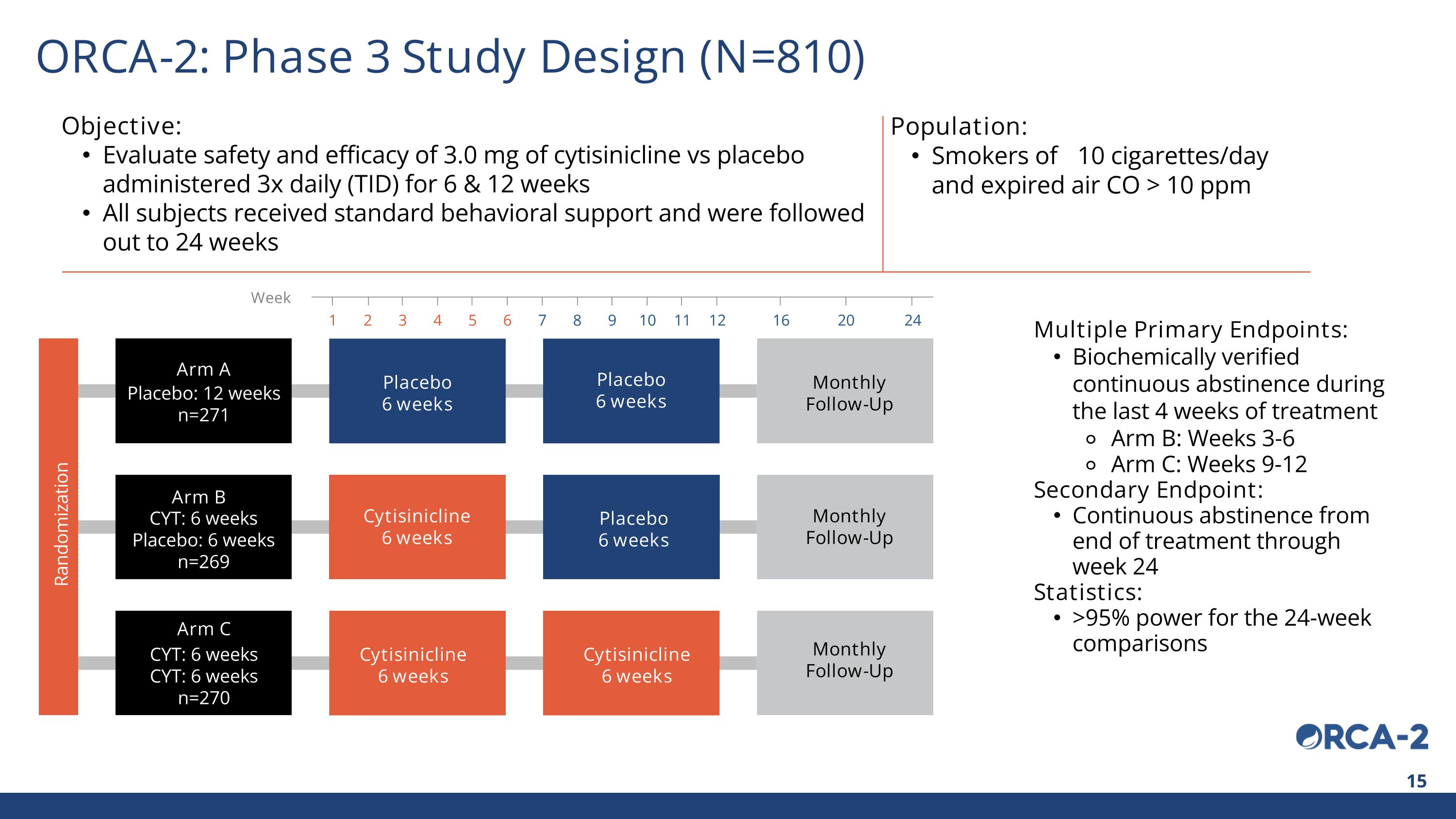
Arm C Arm B Arm A Placebo 6 weeks Cytisinicline 6 weeks Cytisinicline 6 weeks Placebo 6 weeks Placebo 6 weeks Cytisinicline 6 weeks Monthly Follow-Up CYT: 6 weeks CYT: 6 weeks n=270 CYT: 6 weeks Placebo: 6 weeks n=269 Placebo: 12 weeks n=271 Objective: Evaluate safety and efficacy of 3.0 mg of cytisinicline vs placebo administered 3x daily (TID) for 6 & 12 weeks All subjects received standard behavioral support and were followed out to 24 weeks Population: Smokers of ≥10 cigarettes/day and expired air CO > 10 ppm Multiple Primary Endpoints: Biochemically verified continuous abstinence during the last 4 weeks of treatment Arm B: Weeks 3-6 Arm C: Weeks 9-12 Secondary Endpoint: Continuous abstinence from end of treatment through week 24 Statistics: >95% power for the 24-week comparisons Week 1 2 3 4 5 6 7 8 9 10 11 12 16 20 24 Randomization ORCA-2: Phase 3 Study Design (N=810) Monthly Follow-Up Monthly Follow-Up
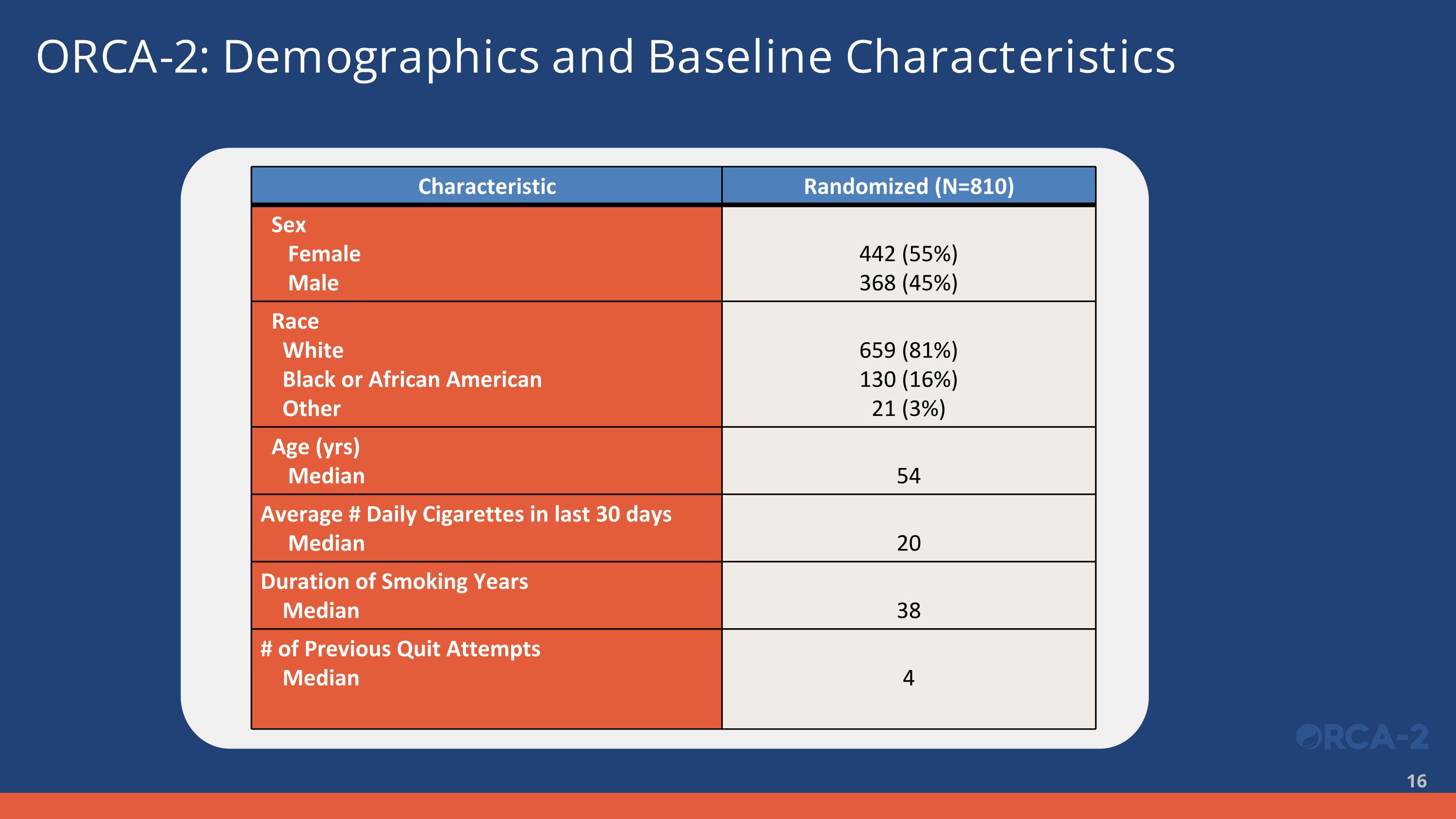
ORCA-2: Demographics and Baseline Characteristics Characteristic Randomized (N=810) Sex Female Male 442 (55%) 368 (45%) Race White Black or African American Other 659 (81%) 130 (16%) 21 (3%) Age (yrs) Median 54 Average # Daily Cigarettes in last 30 days Median 20 Duration of Smoking Years Median 38 # of Previous Quit Attempts Median 4
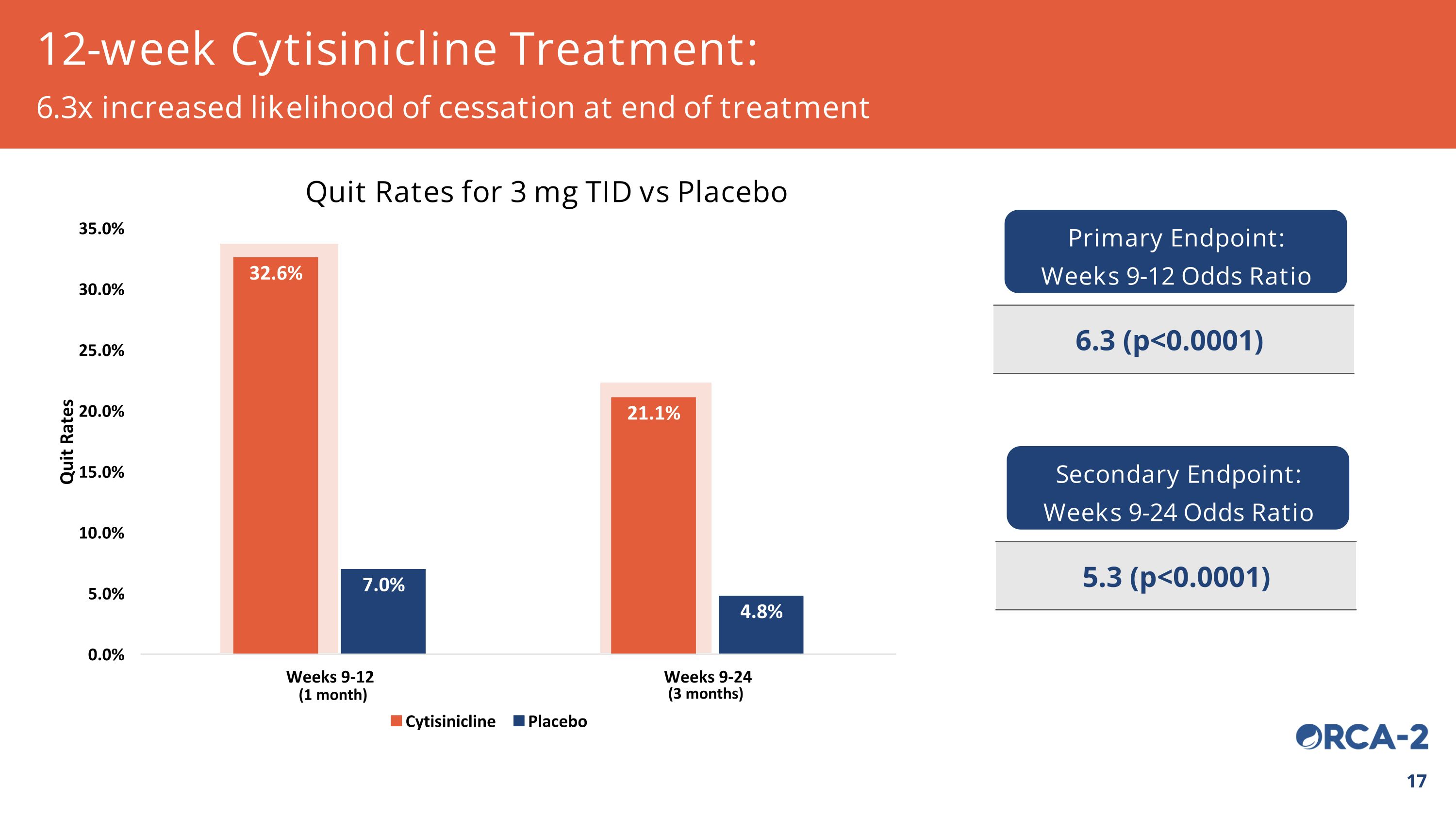
12-week Cytisinicline Treatment: 6.3x increased likelihood of cessation at end of treatment Quit Rates for 3 mg TID vs Placebo Primary Endpoint: Weeks 9-12 Odds Ratio 6.3 (p<0.0001) Secondary Endpoint: Weeks 9-24 Odds Ratio 5.3 (p<0.0001) (3 months) (1 month)
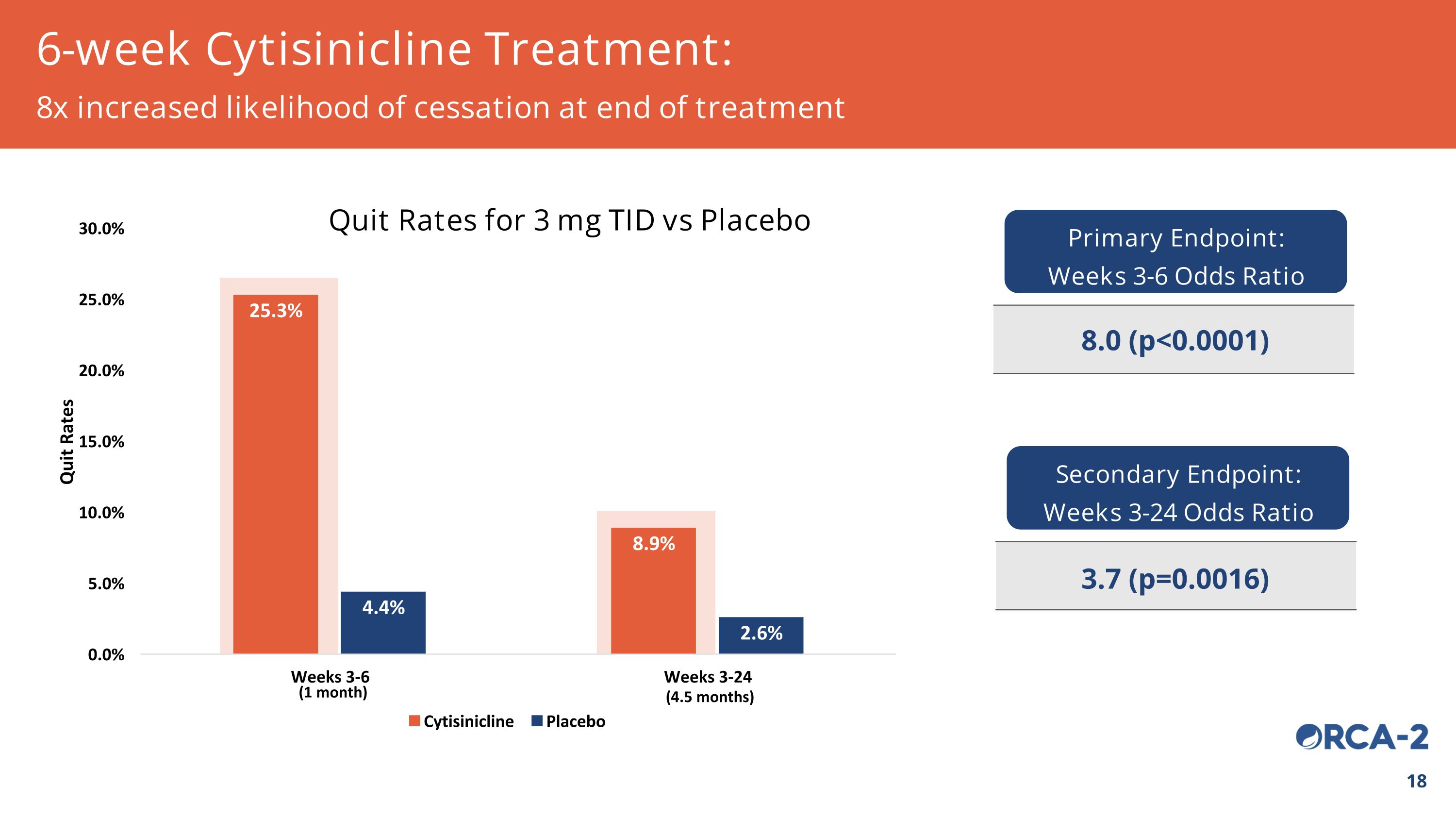
(4.5 months) (1 month) 6-week Cytisinicline Treatment: 8x increased likelihood of cessation at end of treatment Quit Rates for 3 mg TID vs Placebo Primary Endpoint: Weeks 3-6 Odds Ratio 8.0 (p<0.0001) Secondary Endpoint: Weeks 3-24 Odds Ratio 3.7 (p=0.0016)
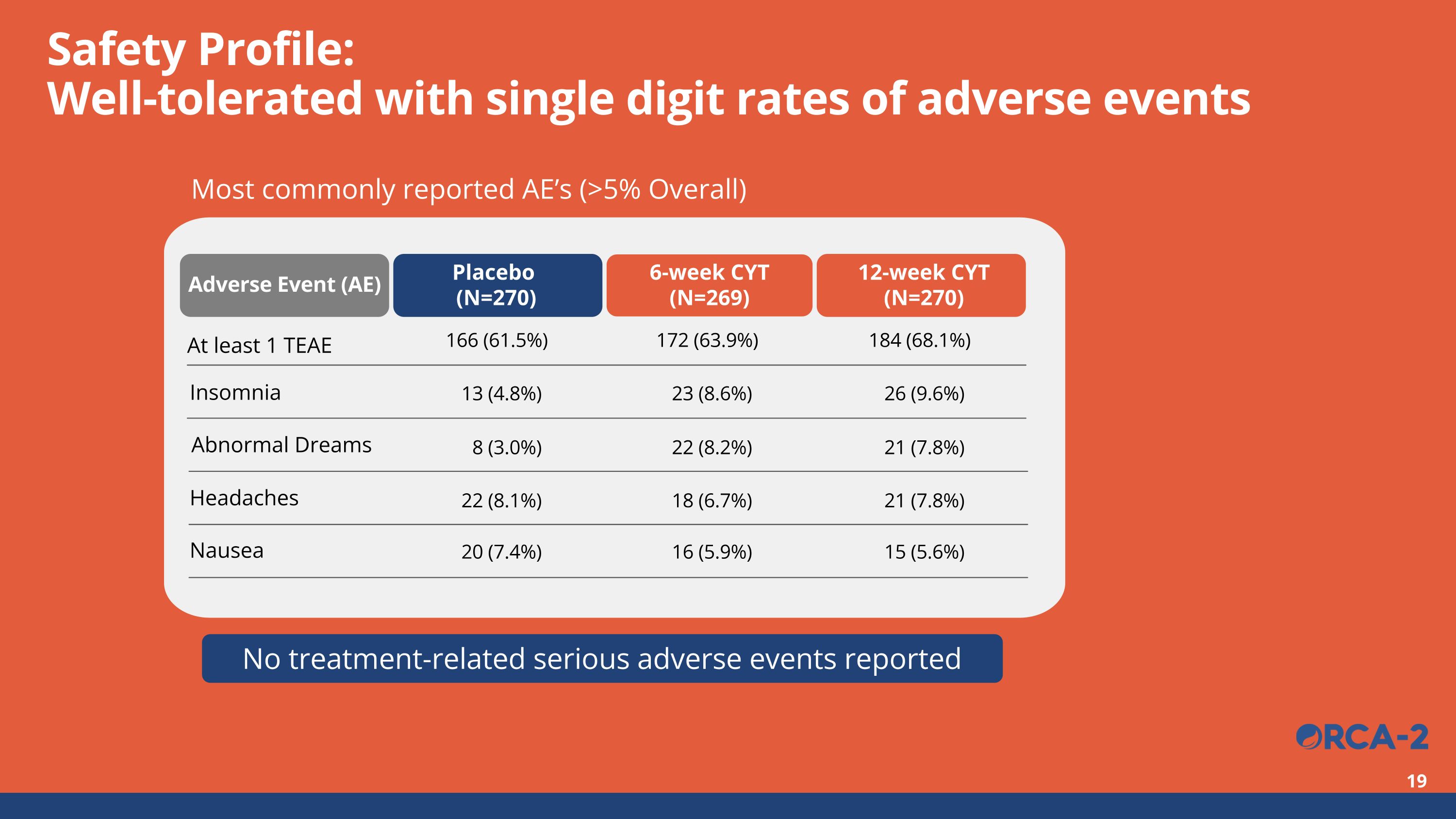
No treatment-related serious adverse events reported Safety Profile:Well-tolerated with single digit rates of adverse events Most commonly reported AE’s (>5% Overall) 166 (61.5%) 172 (63.9%) 184 (68.1%) 13 (4.8%) 23 (8.6%) 26 (9.6%) Adverse Event (AE) Placebo (N=270) 6-week CYT (N=269) 12-week CYT (N=270) At least 1 TEAE Insomnia 8 (3.0%) 22 (8.2%) 21 (7.8%) 22 (8.1%) 18 (6.7%) 21 (7.8%) 20 (7.4%) 16 (5.9%) 15 (5.6%) Nausea Headaches Abnormal Dreams

Forward Looking Statements ORCA-2 & ORCA-3 Combined Results
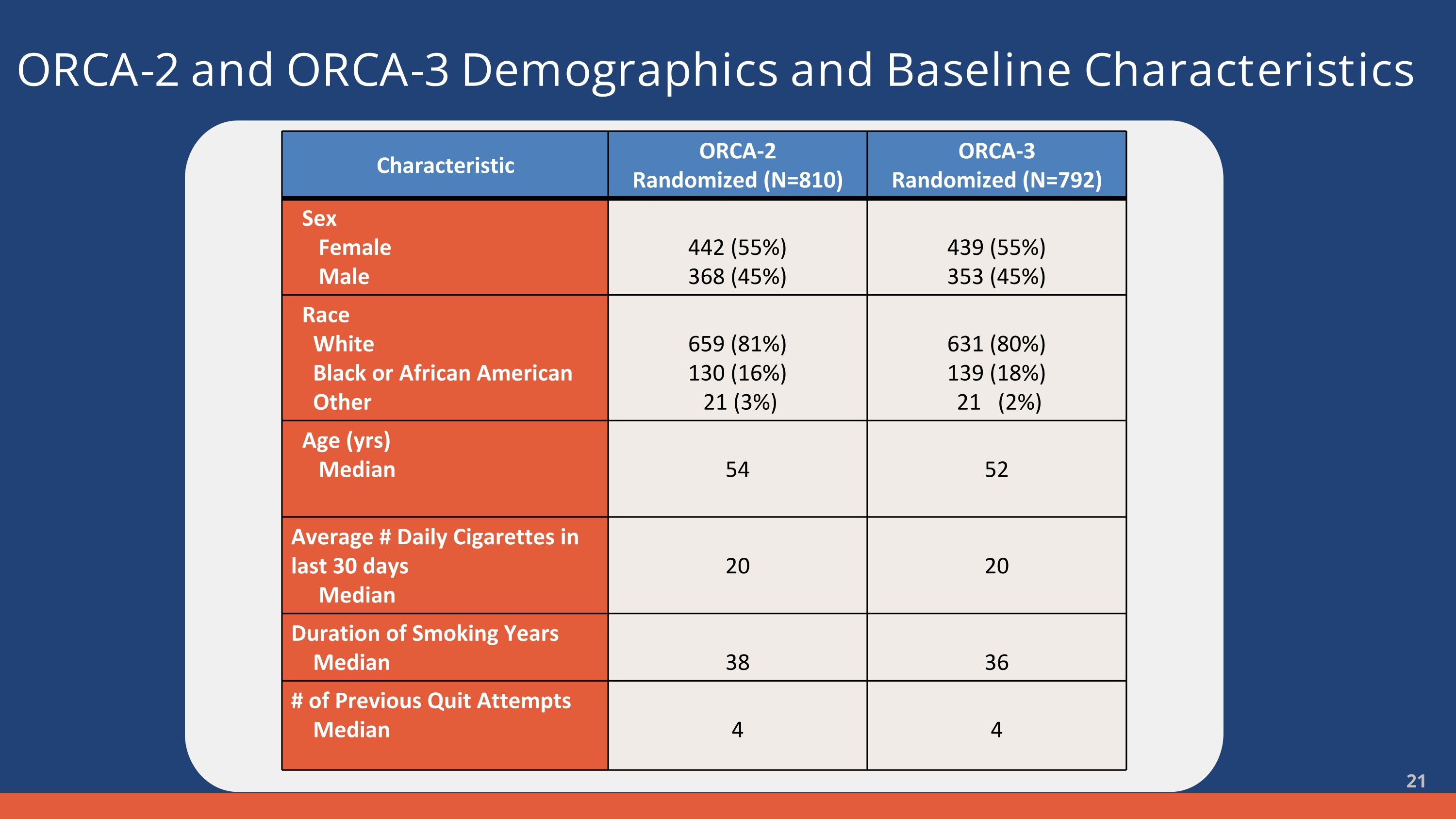
ORCA-2 and ORCA-3 Demographics and Baseline Characteristics Characteristic ORCA-2 Randomized (N=810) ORCA-3 Randomized (N=792) Sex Female Male 442 (55%) 368 (45%) 439 (55%) 353 (45%) Race White Black or African American Other 659 (81%) 130 (16%) 21 (3%) 631 (80%) 139 (18%) 21 (2%) Age (yrs) Median 54 52 Average # Daily Cigarettes in last 30 days Median 20 20 Duration of Smoking Years Median 38 36 # of Previous Quit Attempts Median 4 4
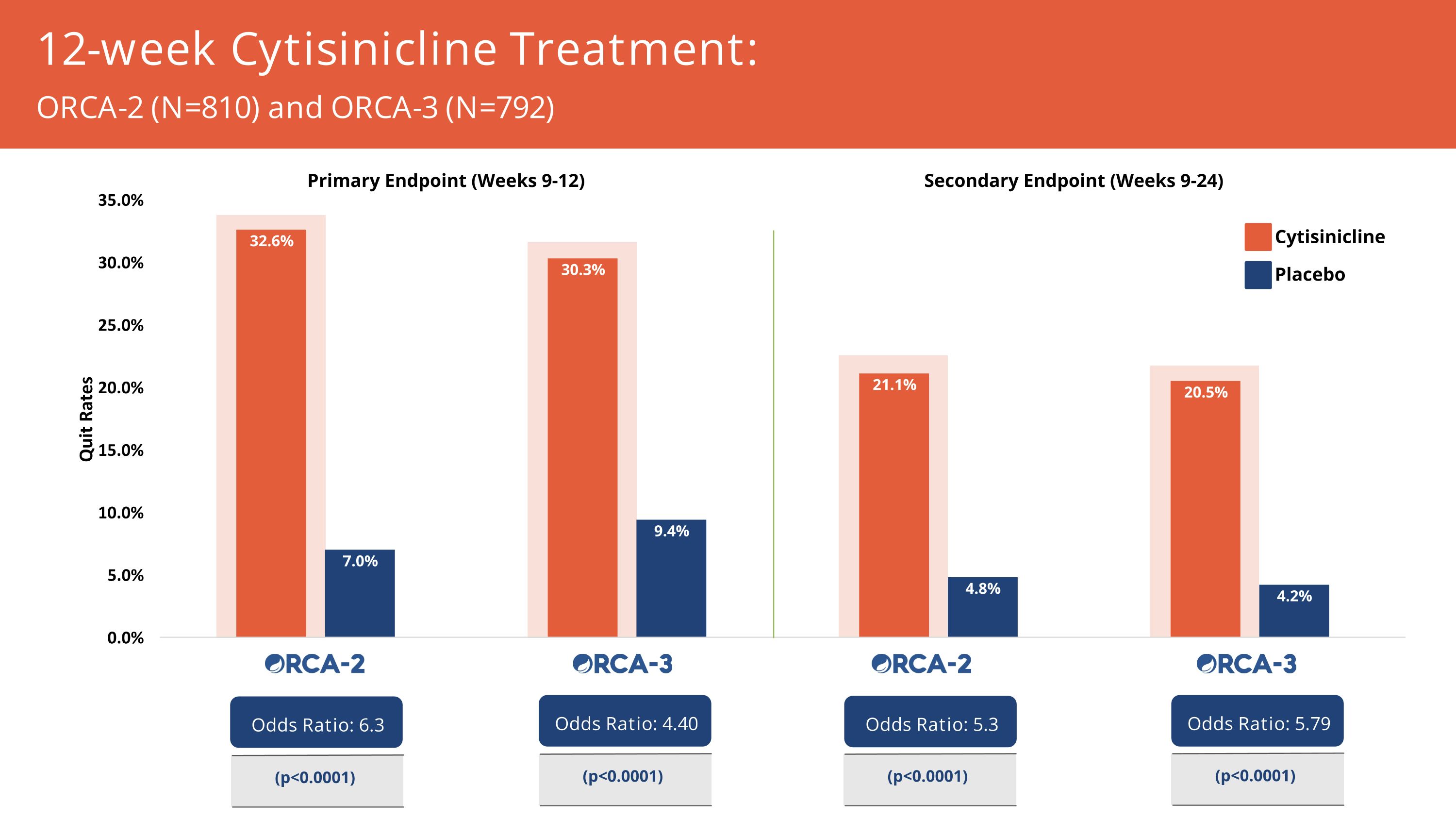
Cytisinicline Placebo 12-week Cytisinicline Treatment: ORCA-2 (N=810) and ORCA-3 (N=792) Secondary Endpoint (Weeks 9-24) Primary Endpoint (Weeks 9-12) Odds Ratio: 5.79 (p<0.0001) Odds Ratio: 5.3 (p<0.0001) Odds Ratio: 4.40 (p<0.0001) Odds Ratio: 6.3 (p<0.0001)
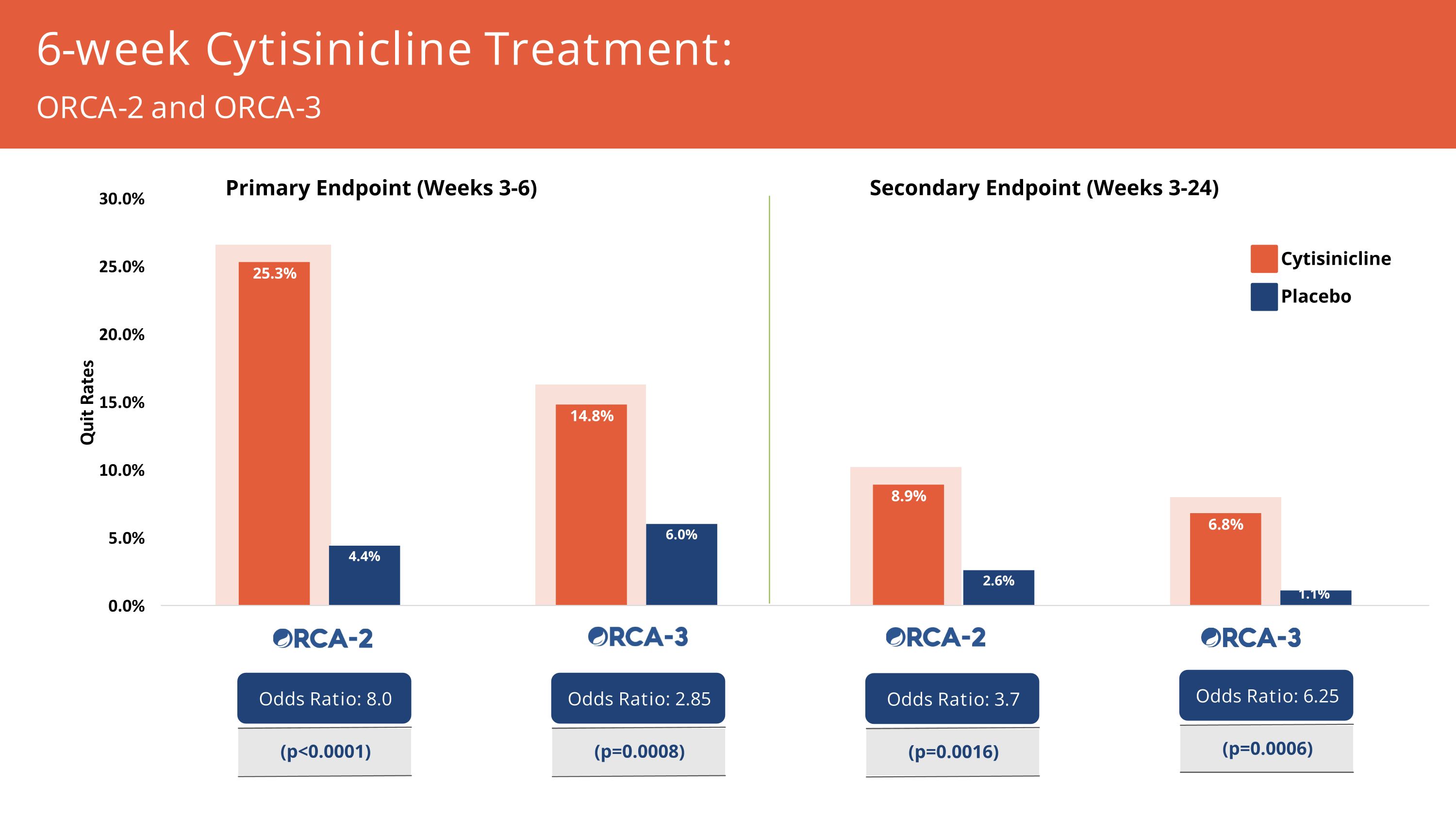
Cytisinicline Placebo 6-week Cytisinicline Treatment: ORCA-2 and ORCA-3 Secondary Endpoint (Weeks 3-24) Primary Endpoint (Weeks 3-6) Odds Ratio: 6.25 (p=0.0006) Odds Ratio: 3.7 (p=0.0016) Odds Ratio: 2.85 (p=0.0008) Odds Ratio: 8.0 (p<0.0001)
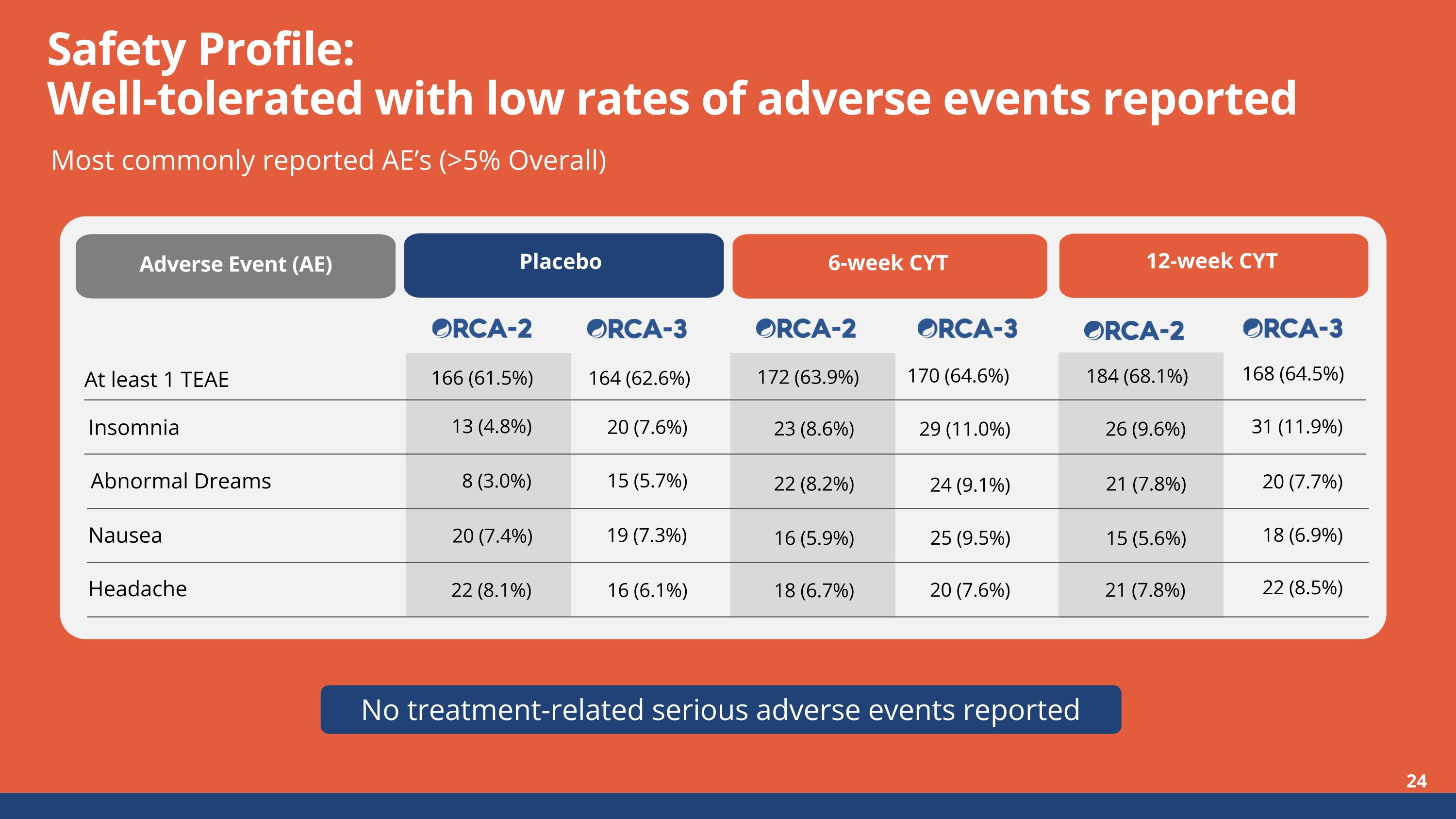
No treatment-related serious adverse events reported Safety Profile:Well-tolerated with low rates of adverse events reported Most commonly reported AE’s (>5% Overall) 166 (61.5%) 172 (63.9%) 184 (68.1%) 13 (4.8%) 23 (8.6%) 26 (9.6%) Adverse Event (AE) Placebo 6-week CYT 12-week CYT At least 1 TEAE Insomnia 8 (3.0%) 22 (8.2%) 21 (7.8%) 20 (7.4%) 16 (5.9%) 15 (5.6%) 22 (8.1%) 18 (6.7%) 21 (7.8%) Headache Nausea Abnormal Dreams 164 (62.6%) 20 (7.6%) 15 (5.7%) 19 (7.3%) 16 (6.1%) 170 (64.6%) 29 (11.0%) 24 (9.1%) 25 (9.5%) 20 (7.6%) 168 (64.5%) 31 (11.9%) 20 (7.7%) 18 (6.9%) 22 (8.5%)