10-Q: Quarterly report pursuant to Section 13 or 15(d)
Published on May 9, 2023
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM
FOR THE QUARTERLY PERIOD ENDED
or
FOR THE TRANSITION PERIOD FROM ______________ TO ____________.
Commission file number
(Exact Name of Registrant as Specified in Its Charter)
|
||
(State or Other Jurisdiction of |
|
(I.R.S. Employer |
Incorporation or Organization) |
|
Identification Number) |
(Address of Principal Executive Offices)
(
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: |
|
||
Title of each class |
Trading Symbol |
Name of exchange on which registered |
|
The |
|
||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
|
|
|
|
|
☒ |
|
Smaller reporting company |
||
|
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date.
As of May 9, 2023 there were
Achieve Life Sciences, Inc.
Index to Form 10-Q
|
Page Number |
|
|
|
|
5 |
||
|
|
|
Item 1 |
5 |
|
|
|
|
|
Consolidated Balance Sheets as of March 31, 2023 (unaudited) and December 31, 2022 |
5 |
|
|
|
|
6 |
|
|
|
|
|
7 |
|
|
|
|
|
8 |
|
|
|
|
|
9 |
|
|
|
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
21 |
|
|
|
|
|
|
Item 4. |
31 |
|
|
|
|
32 |
||
|
|
|
Item 1A. |
32 |
|
|
|
|
Item 6. |
61 |
|
|
|
|
Items 2, 3 and 4 are not applicable and therefore have been omitted. |
|
|
|
|
|
62 |
||
2
INFORMATION REGARDING FORWARD LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve a number of risks and uncertainties. We caution readers that any forward-looking statement is not a guarantee of future performance and that actual results could differ materially from those contained in the forward-looking statement. These statements are based on current expectations of future events. Such statements include, but are not limited to, statements about future financial and operating results, plans, objectives, expectations and intentions, costs and expenses, interest rates, outcome of contingencies, financial condition, results of operations, liquidity, business strategies, cost savings, objectives of management and other statements that are not historical facts. You can find many of these statements by looking for words like “believes,” “expects,” “anticipates,” “estimates,” “may,” “should,” “will,” “could,” “plan,” “intend” or similar expressions in this Quarterly Report on Form 10-Q or in documents incorporated by reference into this Quarterly Report on Form 10-Q. We intend that such forward-looking statements be subject to the safe harbors created thereby. Examples of these forward-looking statements include, but are not limited to:
These forward-looking statements are based on the current beliefs and expectations of our management and are subject to significant risks and uncertainties. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results may differ materially from current expectations and projections. Factors that might cause such a difference include those discussed in Item 1A “Risk Factors,” as well as those discussed elsewhere in the Quarterly Report on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this Quarterly Report on Form 10-Q or, in the case of documents referred to or incorporated by reference, the date of those documents.
All subsequent written or oral forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section. We do not undertake any obligation to release publicly any revisions to these forward-looking statements to reflect events or circumstances after the date of this Quarterly Report on Form 10-Q or to reflect the occurrence of unanticipated events, except as may be required under applicable U.S. securities law. If we do update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
Summary of Risk Factors
An investment in our common stock involves various risks, and prospective investors are urged to carefully consider the matters discussed in the section titled “Risk Factors” prior to making an investment in our common stock. These risks include, but are not limited to, the following:
3
4
PART I. FINANCIAL INFORMATION
Item 1. Consolidated Financial Statements
Achieve Life Sciences, Inc.
Consolidated Balance Sheets
(Unaudited)
(In thousands, except per share and share amounts)
|
|
March 31, |
|
|
December 31, |
|
||
|
|
2023 |
|
|
2022 |
|
||
|
|
|
|
|
|
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents [note 6] |
|
$ |
|
|
$ |
|
||
Grant receivable [note 3] |
|
|
|
|
|
|
||
Prepaid expenses and other assets |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
Right-of-use assets [note 9] |
|
|
|
|
|
|
||
Other assets and restricted cash [note 6] |
|
|
|
|
|
|
||
License agreement [note 4 and note 5] |
|
|
|
|
|
|
||
Goodwill [note 5] |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Accrued liabilities other |
|
|
|
|
|
|
||
Accrued clinical liabilities |
|
|
|
|
|
|
||
Accrued compensation |
|
|
|
|
|
|
||
Current portion of long-term obligations [note 9] |
|
|
|
|
|
|
||
Convertible debt [note 6 and note 7] |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
Long-term obligations [note 9] |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
[note 9] |
|
|
|
|
|
|
||
Stockholders' equity: |
|
|
|
|
|
|
||
Series A convertible preferred stock, $ |
|
|
|
|
|
|
||
Series B convertible preferred stock, $ |
|
|
|
|
|
|
||
Common stock, $ |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Accumulated other comprehensive income |
|
|
|
|
|
|
||
Total stockholders' equity |
|
|
|
|
|
|
||
Total liabilities and stockholders' equity |
|
$ |
|
|
$ |
|
||
Going concern [note 1] |
|
|
|
|
|
|
||
See accompanying notes.
5
Achieve Life Sciences, Inc.
Consolidated Statements of Loss and Comprehensive Loss
(Unaudited)
(In thousands, except per share and share amounts)
|
|
Three Months Ended |
|
|||||
|
|
March 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
EXPENSES |
|
|
|
|
|
|
||
Research and development |
|
|
|
|
|
|
||
General and administrative |
|
|
|
|
|
|
||
Total operating expenses |
|
|
|
|
|
|
||
OTHER INCOME (EXPENSE) |
|
|
|
|
|
|
||
Interest income |
|
|
|
|
|
|
||
Interest expense [note 7] |
|
|
( |
) |
|
|
( |
) |
Other income (expense) |
|
|
( |
) |
|
|
|
|
Total other (expense) |
|
|
( |
) |
|
|
( |
) |
Net loss and comprehensive loss |
|
$ |
( |
) |
|
$ |
( |
) |
Basic and diluted net loss per common share [note 8[d]] |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted average shares used in computation of basic and diluted net loss per common share [note 8[d]] |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
See accompanying notes.
6
Achieve Life Sciences, Inc.
Consolidated Statements of Cash Flows
(Unaudited)
(In thousands)
|
|
Three Months Ended |
|
|||||
|
|
March 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Operating Activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Depreciation and amortization [note 4] |
|
|
|
|
|
|
||
Stock-based compensation [note 8[c], note 8[e], note 8[f] and note 8[g]] |
|
|
|
|
|
|
||
Shares issued as settlement with trade vendor |
|
|
— |
|
|
|
|
|
Accrued interest on SVB convertible debt [note 7] |
|
|
|
|
|
|
||
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Grant receivable [note 3] |
|
|
|
|
|
|
||
Prepaid expenses and other assets |
|
|
|
|
|
( |
) |
|
Accounts payable |
|
|
( |
) |
|
|
( |
) |
Accrued liabilities other |
|
|
|
|
|
|
||
Accrued clinical liabilities |
|
|
( |
) |
|
|
|
|
Accrued compensation |
|
|
( |
) |
|
|
( |
) |
Lease obligation [note 9] |
|
|
( |
) |
|
|
|
|
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
Financing Activities: |
|
|
|
|
|
|
||
Proceeds from exercise of warrants |
|
|
|
|
|
|
||
Proceeds from ATM, net of issuance costs |
|
|
— |
|
|
|
|
|
Financing costs relating to convertible debt with SVB |
|
|
— |
|
|
|
( |
) |
Financing costs relating to November 2022 private placement |
|
|
( |
) |
|
|
— |
|
Net cash provided by financing activities |
|
|
|
|
|
|
||
Effect of exchange rate changes on cash |
|
|
( |
) |
|
|
( |
) |
Net decrease in cash, cash equivalents and restricted cash |
|
|
( |
) |
|
|
( |
) |
Cash, cash equivalents and restricted cash at beginning of the period |
|
|
|
|
|
|
||
Cash, cash equivalents and restricted cash at end of the period |
|
$ |
|
|
$ |
|
||
See accompanying notes.
7
Achieve Life Sciences, Inc.
Consolidated Statements of Stockholders’ Equity
(Unaudited)
(In thousands, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Other |
|
|
|
|
|
Total, |
|
||||||||
|
|
Common Stock |
|
|
Preferred Stock |
|
|
Paid-in |
|
|
Comprehensive |
|
|
Accumulated |
|
|
Stockholders’ |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income (Loss) |
|
|
Deficit |
|
|
Equity |
|
||||||||
Balance, December 31, 2022 |
|
|
|
|
$ |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
|
|
$ |
|
|
$ |
( | ) |
|
$ |
|
|||||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Shares issued on exercise of warrants |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Financing costs relating to November 2022 private placement |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance, March 31, 2023 |
|
|
|
|
$ |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
|
|
$ |
|
|
$ |
( | ) |
|
$ |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Other |
|
|
|
|
|
Total, |
|
||||||||
|
|
Common Stock |
|
|
Preferred Stock |
|
|
Paid-in |
|
|
Comprehensive |
|
|
Accumulated |
|
|
Stockholders’ |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income (Loss) |
|
|
Deficit |
|
|
Equity |
|
||||||||
Balance, December 31, 2021 |
|
|
|
|
$ |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Shares issued on exercise of warrants |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Shares issued from purchase agreement with Virtu |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Shares issued as settlement with trade vendor |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance, March 31, 2022 |
|
|
|
|
$ |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
|
|
$ |
|
|
$ |
( | ) |
|
$ |
|
|||||
See accompanying notes.
8
Achieve Life Sciences, Inc.
Notes to Consolidated Financial Statements
(Unaudited)
1. NATURE OF BUSINESS, BASIS OF PRESENTATION AND GOING CONCERN UNCERTAINTY
Achieve Life Sciences, Inc. (referred to as “Achieve,” “we,” “us,” or “our”) is a clinical-stage pharmaceutical company committed to the global development and commercialization of cytisinicline for smoking cessation and nicotine addiction. We were incorporated in the state of Delaware, and operate out of Seattle, Washington and Vancouver, British Columbia.
The unaudited consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP, for interim financial information and with the instructions to Form 10-Q. Accordingly, they do not include all of the information and footnotes required to be presented for complete financial statements. The accompanying unaudited consolidated financial statements reflect all adjustments (consisting only of normal recurring items) which are, in the opinion of management, necessary for a fair presentation of the results for the interim periods presented. The accompanying consolidated Balance Sheet at December 31, 2022 has been derived from the audited consolidated financial statements included in our Annual Report on Form 10-K for the year then ended. The unaudited consolidated financial statements and related disclosures have been prepared with the assumption that users of the interim financial information have read or have access to the audited consolidated financial statements for the preceding fiscal year. Accordingly, these financial statements should be read in conjunction with the audited consolidated financial statements and the related notes thereto included in the Annual Report on Form 10-K for the year ended December 31, 2022 and filed with the U.S. Securities and Exchange Commission, or the SEC, on March 16, 2023.
The consolidated financial statements include the accounts of Achieve and our wholly owned subsidiaries, Achieve Life Sciences Technologies Inc., Achieve Life Science, Inc., Extab Corporation, and Achieve Pharma UK Limited. All intercompany balances and transactions have been eliminated.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming we will continue to operate as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business.
We have historically experienced recurring losses from operations and have incurred an accumulated deficit of $
Substantial doubt exists as to our ability to continue as a going concern. Our ability to continue as a going concern is subject to material uncertainty and dependent on our ability to obtain additional financing. We have historically financed our operations through equity and debt financings. There can be no assurance that financing from these or other sources will be available to us in the future. Without additional funds, we may be forced to delay, scale back or eliminate some of our research and development, or R&D, activities or other operations and potentially delay product development in an effort to provide sufficient funds to continue our operations. If any of these events occurs, our ability to achieve our development and commercialization goals would be adversely affected.
Our current resources are insufficient to fund our planned operations for the next twelve months. We will continue to require substantial additional capital to continue our clinical development activities. Accordingly, we will need to raise substantial additional capital to continue to fund our operations from the sale of our securities, debt, partnering arrangements, non-dilutive fundraising or other financing transactions in order to finance the remaining development and commercialization of our product candidate. The amount and timing of our future funding requirements will depend on many factors, including the pace and results of our clinical development efforts. The uncertainty with respect to our operations and the market generally due to increasing interest rates and inflation may also make it challenging to raise additional capital on favorable terms, if at all. In addition, current macroeconomic conditions have caused turmoil in the banking sector. For example, on March 10, 2023, Silicon Valley Bank, or SVB, one of our banking partners, was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation, or FDIC, as receiver. On March 26, 2023, it was announced that First-Citizens Bank & Trust Company would assume all of SVB’s deposits and loans as of March 27, 2023 and since that date we continue to have full access to our cash and cash equivalents. Failure to raise capital as and when needed, on favorable terms or at all, will have a negative impact on our financial condition and our ability to develop our product candidate. We expect our R&D expenses to substantially increase in connection with our ongoing activities, particularly as we advance our product candidate in clinical development.
9
As disclosed in Note 7, we are required to keep substantially all of our cash and cash equivalents with a single financial institution, SVB, as required by the covenants of our Debt Agreement (Note 7 – Convertible Debt), and we have a loan and security agreement, or Loan Agreement, with SVB under which we have the option to borrow up to $
The consolidated financial statements do not include any adjustments to the amounts and classification of assets and liabilities that might be necessary should we be unable to continue as a going concern. Such adjustments could be material.
2. ACCOUNTING POLICIES
The preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect reported amounts and related disclosures. We have discussed those estimates that we believe are critical and require the use of complex judgment in their application in our audited financial statements for the year ended December 31, 2022 in our Annual Report on Form 10-K filed with the SEC, on March 16, 2023. Since December 31, 2022, there have been no material changes to our critical accounting policies or the methodologies or assumptions we apply under them.
3. GOVERNMENT GRANT
In July 2021, we announced that we were awarded a grant from the National Institute on Drug Abuse, or NIDA, of the National Institutes of Health, or NIH, to evaluate the use of cytisinicline as a treatment for cessation of nicotine e-cigarette use. This initial grant award, in the amount of $
In November 2021, we announced that the FDA had completed their review and accepted the IND application to investigate cytisinicline as a cessation treatment in this population. In June 2022, following NIH review of completed milestones, we announced that we were awarded the next grant funding from the NIDA in the amount of approximately $
In June 2022, we announced the initiation of the ORCA-V1 Phase 2 clinical trial. ORCA-V1 will evaluate the efficacy and safety of 3 mg cytisinicline dosed three times daily compared to placebo in approximately 160 adult e-cigarette users at five clinical trial locations in the United States. Participants were randomized to receive cytisinicline or placebo for 12 weeks in combination with standard cessation behavioral support.
The full grant award of $
For the three months ended March 31, 2023, we incurred $
As of March 31, 2023, we had $
4. INTANGIBLES
All of our intangible assets are subject to amortization and are amortized using the straight-line method over their estimated useful life.
We acquired license and supply agreements in relation to cytisinicline upon the acquisition of Extab Corporation, or Extab, on May 18, 2015. The agreements were determined to have a fair value of $
The components of intangible assets were as follows:
10
|
|
March 31, 2023 |
|
|
December 31, 2022 |
|
||||||||||||||||||
|
|
Gross Carrying |
|
|
Accumulated |
|
|
Net Carrying |
|
|
Gross Carrying |
|
|
Accumulated |
|
|
Net Carrying |
|
||||||
|
|
Value |
|
|
Amortization |
|
|
Value |
|
|
Value |
|
|
Amortization |
|
|
Value |
|
||||||
License Agreements |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||||
For each of the three months ended March 31, 2023 and 2022, we recorded license agreement amortization expense of $
Year Ending December 31, |
|
|
|
|
2023 |
|
|
|
|
2024 |
|
|
|
|
2025 |
|
|
|
|
Thereafter |
|
|
|
|
Total |
|
$ |
|
|
We evaluate the carrying amount of intangible assets periodically by taking into account events or circumstances that may warrant revised estimates of useful life or that indicate the asset may be impaired. We conducted an analysis of potential impairment indicators for long lived assets, including the license and supply agreements for the active pharmaceutical ingredient cytisinicline, and concluded that there were
5. LICENSE AGREEMENTS
Sopharma License and Supply Agreements
We are party to a license agreement, or the Sopharma License Agreement, and a supply agreement, or the Sopharma Supply Agreement, with Sopharma, AD, or Sopharma. Pursuant to the Sopharma License Agreement, we were granted access to all available manufacturing, efficacy and safety data related to cytisinicline, as well as a granted patent in several European countries related to new oral dosage forms of cytisinicline providing enhanced stability. Additional rights granted under the Sopharma License Agreement include the exclusive use of, and the right to sublicense, certain cytisinicline trademarks in all territories described in the Sopharma License Agreement. Under the Sopharma License Agreement, we agreed to pay a nonrefundable license fee. In addition, we agreed to make certain royalty payments equal to a mid-single digit percentage of all net sales of cytisinicline products in our territory during the term of the Sopharma License Agreement, including those sold by a third party pursuant to any sublicense which may be granted by us. To date, any amounts paid to Sopharma pursuant to the Sopharma License Agreement have been immaterial.
6. FAIR VALUE MEASUREMENTS
Assets and liabilities recorded at fair value in the balance sheets are categorized based upon the level of judgment associated with the inputs used to measure their fair value. For certain of our financial instruments including amounts receivable and accounts payable the carrying values approximate fair value due to their short-term nature.
ASC 820 “Fair Value Measurements and Disclosures” specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. In accordance with ASC 820, these inputs are summarized in the three broad levels listed below:
As quoted prices in active markets are not readily available for certain financial instruments, we obtain estimates for the fair value of financial instruments through third-party pricing service providers.
In determining the appropriate levels, we performed a detailed analysis of the assets and liabilities that are subject to ASC 820.
We invest our excess cash in accordance with investment guidelines that limit the credit exposure to any one financial institution other than securities issued by the U.S. Government. These securities are not collateralized and mature within
A description of the valuation techniques applied to our financial instruments measured at fair value on a recurring basis follows.
11
Financial Instruments
Money Market Securities
Money market securities are classified within Level 1 of the fair value hierarchy and are valued based on quoted prices in active markets for identical securities.
The following table presents information about our assets that are measured at fair value on a recurring basis, and indicates the fair value hierarchy of the valuation techniques we utilized to determine such fair value (in thousands):
March 31, 2023 |
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market securities (cash equivalents) |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Restricted cash |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Cash equivalents consist of the following (in thousands):
|
|
|
|
|
Gross |
|
|
Gross |
|
|
|
|
||||
|
|
Amortized |
|
|
Unrealized |
|
|
Unrealized |
|
|
Estimated |
|
||||
March 31, 2023 |
|
Cost |
|
|
Gains |
|
|
Losses |
|
|
Fair Value |
|
||||
Money market securities |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Total cash equivalents |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
Money market securities (restricted cash) |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Total restricted cash |
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||
We only invest in A (or equivalent) rated securities. All securities included in cash and cash equivalents had maturities of
Concentration of Cash and Cash Equivalents Risk
We place our cash primarily in commercial checking accounts with various financial institutions. As of March 31, 2023, approximately $
Fair Value of Long-Term Debt
December 2021 Convertible Debt
The principal amount, carrying value and related estimated fair value of our convertible debt reported in the consolidated balance sheets as of March 31, 2023 and December 31, 2022 was as follows (in thousands).
|
|
March 31, 2023 |
|
|
December 31, 2022 |
|
||||||||||||||||||
|
|
Principal |
|
|
Carrying |
|
|
Fair |
|
|
Principal |
|
|
Carrying |
|
|
Fair |
|
||||||
|
|
Amount |
|
|
Value |
|
|
Value |
|
|
Amount |
|
|
Value |
|
|
Value |
|
||||||
December 2021 Convertible Debt |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||
7. CONVERTIBLE DEBT
On December 22, 2021, we entered into a $
12
Under the terms of the Debt Agreement, the Convertible Debt matures on
Subject to certain terms and conditions, the Lenders may convert all or any part of the outstanding Convertible Debt and accrued and unpaid interest at any time prior to maturity into shares of our common stock at a conversion price equal to $
We have the right, or Call Right, at any time to repay and retire all (but not less than all) of the outstanding Convertible Debt and accrued and unpaid interest, if any, prior to its conversion by payment of a premium determined based on the date of such repayment equal to:
in either case together with all accrued and unpaid interest on the principal balance of the Convertible Debt. If the Call Right is exercised by us, the Lenders will retain certain lookback rights in the event we enter into an agreement to be acquired in the twelve months following the exercise of the Call Right. We agreed to grant the Lenders a security interest in virtually all of our assets, including our patents and other intellectual property as security for our obligations under the Debt Agreement.
Subject to the terms and conditions of the Loan Agreement, we could borrow Term Loans under the Loan Agreement until April 30, 2023. Amounts borrowed under the Loan Agreement incurred interest at a floating rate equal to the greater of
Upon and after borrowing under the Loan Agreement, we must comply with certain financial covenants as set forth in the Loan Agreement and the Amendment, including a minimum liquidity ratio of at least
Under ASU 2020-06, the embedded conversion feature was not required to be bifurcated and recognized separately, as a result the convertible debt including the conversion feature has been recognized as a single unit of debt. The debt issuance costs have been recognized against the single unit of debt and will be amortized into interest expense over the term of the loan.
As of March 31, 2023 and December 31, 2022, the Convertible Debt balance was comprised of the following:
|
March 31, |
|
|
December 31, |
|
||
|
2023 |
|
|
2022 |
|
||
Convertible Debt Information |
|
|
|
|
|
||
Principal |
$ |
|
|
$ |
|
||
Transaction Costs |
|
( |
) |
|
|
( |
) |
Accrued paid-in-kind interest |
|
|
|
|
|
||
|
|
|
|
|
|
||
13
8. COMMON STOCK
[a] Authorized
[b] Issued and outstanding shares
At-the-Market Sales Agreement
On December 21, 2021, we entered into an At-the-Market Offering Sales Agreement, or ATM, with Virtu Americas, LLC, as sales agent, pursuant to which we may sell shares of common stock with an aggregate offering price of up to $
Since entry into the ATM, from December 21, 2021 through March 31, 2023, we offered and sold an aggregate of
During the three months ended March 31, 2023, we did
November 2022 Private Placement
In November 2022, we entered into subscription agreements with certain accredited investors pursuant to which we sold to the purchasers in a private placement transaction approximately
The November 2022 Warrants are exercisable at a price per share of common stock of $
We received approximately $
Equity Award Issuances and Settlements
During the three months ended March 31, 2023 and 2022, we did
14
[c] Stock options
2018 Equity Incentive Plan
As of March 31, 2023, we had reserved, pursuant to the 2018 Equity Incentive Plan, or the 2018 Plan,
Under the 2018 Plan, we may grant options to purchase common shares or restricted stock units to our employees, directors, officers and consultants. The exercise price of the options is determined by our board of directors, or Board, but will be at least equal to the fair value of the shares of common stock at the grant date. The options vest in accordance with terms as determined by our Board, typically over to
New Employee Inducement Grants
We grant stock options as a material inducement to new employees for entering into employment agreements with us in accordance with Nasdaq Listing Rule 5635(c)(4). The stock options approved under the inducement grants are issued pursuant to a stock option agreement on terms substantially similar to those described in our 2018 Plan. The exercise price of the options is determined by our board of directors but will be at least equal to the fair value of the common shares at the grant date. The options vest in accordance with terms as determined by our board of directors. The expiry date for each option is set by our board of directors with a maximum expiry date of
2017 Equity Incentive Plan
As of March 31, 2023, we had reserved, pursuant to the 2017 Equity Incentive Plan, or the 2017 Plan,
Under the 2017 Plan, we granted options to purchase shares of common stock or restricted stock units to our employees, directors, officers and consultants. The exercise price of the options was determined by our board of directors but was at least equal to the fair value of the shares of common stock at the grant date. The options vest in accordance with terms as determined by our Board, typically over to
2010 Performance Incentive Plan
As of March 31, 2023, we had reserved, pursuant to the 2010 Performance Incentive Plan, or the 2010 Plan,
Under the 2010 Plan we granted options to purchase shares of common stock and restricted stock units to our employees, directors, officers and consultants. The exercise price of the options was determined by our board of directors and was at least equal to the fair value of the shares of common stock at the grant date. The options vest in accordance with terms as determined by our Board, typically over to
15
Stock Option Summary
We grant stock options that vest over time in accordance with terms as determined by our Board, which are typically
Stock option transactions and the number of stock options outstanding are summarized below:
|
|
Number of |
|
|
Weighted |
|
||
|
|
Optioned |
|
|
Average |
|
||
|
|
Common |
|
|
Exercise |
|
||
|
|
Shares |
|
|
Price |
|
||
Balance, December 31, 2022 |
|
|
|
|
$ |
|
||
Granted |
|
|
|
|
|
|
||
Expired |
|
|
( |
) |
|
|
|
|
Balance, March 31, 2023 |
|
|
|
|
$ |
|
||
The fair value of each stock award for employees and directors is estimated on the grant date and for consultants at each reporting period, using the Black-Scholes option-pricing model based on the weighted-average assumptions noted in the following table:
|
|
Three Months Ended |
|
|||||
|
|
March 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Risk-free interest rates |
|
|
% |
|
|
% |
||
Expected dividend yield |
|
|
% |
|
|
% |
||
Expected life |
|
|
|
|
|
|
||
Expected volatility |
|
|
% |
|
|
% |
||
The expected life was calculated based on the simplified method as permitted by the SEC’s Staff Accounting Bulletin 110, Share-Based Payment. We consider the use of the simplified method appropriate because of the lack of sufficient historical exercise data following the Arrangement. The computation of expected volatility was based on the historical volatility of comparable companies from a representative peer group selected based on industry and market capitalization. The risk-free interest rate is based on a U.S. Treasury instrument whose term is consistent with the expected life of the stock options. In addition to the assumptions above, as required under ASC 718, management made an estimate of expected forfeitures and is recognizing compensation costs only for those equity awards expected to vest. Forfeiture rates are estimated using historical actual forfeiture rates. These rates are adjusted on a quarterly basis and any change in compensation expense is recognized in the period of the change. We have never paid or declared cash dividends on our common stock and do not expect to pay cash dividends in the foreseeable future.
The results for the periods set forth below included share-based compensation expense for stock options, restricted stock units and employee share purchase plan compensation expenses in the following expense categories of the consolidated statements of loss (in thousands):
|
|
Three Months Ended |
|
|||||
|
|
March 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Research and development |
|
$ |
|
|
$ |
|
||
General and administrative |
|
$ |
|
|
|
|
||
Total stock-based compensation |
|
$ |
|
|
$ |
|
||
As of March 31, 2023, the total unrecognized compensation expense related to stock options granted was $
16
[d] Loss Per Share
For the three months ended March 31, 2023, a total of
[e] Restricted Stock Unit Awards
We grant restricted stock unit awards that generally vest and are expensed over a
The following table summarizes our restricted stock unit award activity during the three months ended March 31, 2023:
|
|
|
|
|
Weighted |
|
||
|
|
Number |
|
|
Average |
|
||
|
|
of |
|
|
Grant Date |
|
||
|
|
Shares |
|
|
Fair Value |
|
||
Balance, December 31, 2022 |
|
|
|
|
$ |
|
||
Granted |
|
|
|
|
|
|
||
Balance, March 31, 2023 |
|
|
|
|
$ |
|
||
As of March 31, 2023, we had approximately $
[f] Non-employee options and restricted stock units
We recognize non-employee stock-based compensation expense over the period of expected service by the non-employee. As the service is performed, we are required to update our valuation assumptions, re-measure unvested options and restricted stock units and record the stock-based compensation using the valuation as of the vesting date. This differs from the accounting for employee awards where the fair value is determined at the grant date and is not subsequently adjusted. This re-measurement may result in higher or lower stock-based compensation expense in the Consolidated Statements of Loss and Comprehensive Loss. As such, changes in the market price of our stock could materially change the value of an option or restricted stock unit and the resulting stock-based compensation expense.
[g] Employee Share Purchase Plan
Our board of directors and stockholders approved the 2017 Employee Stock Purchase Plan, or ESPP, in August 2017. Contributions are made by eligible employees, subject to certain limits defined in the ESPP. The maximum number of shares authorized to be purchased under the ESPP is
17
[h] Common Stock Warrants
The following is a summary of outstanding warrants to purchase common stock as at March 31, 2023:
|
|
Total |
|
|
|
|
|
|
||
|
|
Outstanding |
|
|
Exercise |
|
|
|
||
|
|
and |
|
|
price per |
|
|
|
||
|
|
Exercisable |
|
|
Share |
|
|
Expiration Date |
||
(1) Warrants issued in June 2018 financing |
|
|
|
|
$ |
|
|
|||
(2) Warrants issued in October 2018 financing |
|
|
|
|
$ |
|
|
|||
(3) Warrants issued in May 2019 financing |
|
|
|
|
$ |
|
|
|||
(4) Warrants issued in December 2019 financing |
|
|
|
|
$ |
|
|
|||
(5) Warrants issued in April 2020 financing |
|
|
|
|
$ |
|
|
|||
(6) Warrants issued in April 2020 financing |
|
|
|
|
$ |
|
|
|||
(7) Warrants issued in April 2020 financing |
|
|
|
|
$ |
|
|
|||
(8) Pre-Funded warrants issued in August 2020 financing |
|
|
|
|
$ |
|
|
* |
||
(9) Warrants issued in December 2020 financing |
|
|
|
|
$ |
|
|
|||
(10) Warrants issued in November 2022 financing |
|
|
|
|
$ |
|
|
|||
*The pre-funded warrants do not have an expiration date.
The agreements governing the above warrants include the following terms:
For the three months ended March 31, 2023, warrants to purchase
18
9. COMMITMENTS AND CONTINGENCIES
The following table summarizes our contractual obligations as of March 31, 2023 (in thousands):
|
|
Total |
|
|
Less than 1 year |
|
|
1-3 years |
|
|
3-5 years |
|
|
More than 5 years |
|
|||||
Vancouver office operating lease |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|||
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|||
Leases
We have operating leases for our corporate offices.
Operating leases with a term of 12 months or longer are included in ROU assets, other current liabilities, and operating lease liabilities on our consolidated balance sheets. Finance leases are included in property and equipment, other current liabilities, and other long-term liabilities on our consolidated balance sheets.
Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. As most of our leases do not provide an implicit rate, we use the incremental borrowing rate of comparable companies from a representative peer group selected based on industry and market capitalization. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term.
Vancouver lease arrangement
On November 19, 2018, we entered into a lease agreement, or the Vancouver Lease, for new office space in Vancouver, British Columbia, which commenced on
Future minimum lease payments under the Vancouver Lease are as follows (in thousands):
2023 |
|
|
|
|
2024 |
|
|
|
|
2025 |
|
|
|
|
Total |
|
$ |
|
Consolidated rent expense relating to the Vancouver, British Columbia office, for the three months ended March 31, 2023 and 2022 was $
Other information related to leases was as follows:
|
|
Three Months Ended |
|
|
|||||
|
|
March 31, |
|
|
|||||
|
|
2023 |
|
|
2022 |
|
|
||
Supplemental Cash Flows Information |
|
|
|
|
|
|
|
||
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|
|
|
|
||
Operating cash flows to operating leases |
|
$ |
|
|
$ |
|
|
||
Right-of-use assets obtained in exchange for lease obligations: |
|
|
|
|
|
|
|
||
Operating leases |
|
|
— |
|
|
|
— |
|
|
Weighted Average Remaining Lease Term |
|
|
|
|
|
|
|
||
Operating leases |
|
|
|
|
|
||||
Weighted Average Discount Rate |
|
|
|
|
|
|
|
||
Operating leases |
|
|
% |
|
|
% |
|
||
19
Guarantees and Indemnifications
We indemnify our officers, directors and certain consultants for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at its request in such capacity. The term of the indemnification period is equal to the officer’s or director’s lifetime.
The maximum amount of potential future indemnification is unlimited; however, we have obtained director and officer insurance that limits our exposure and may enable us to recover a portion of any future amounts paid. We believe that the fair value of these indemnification obligations is minimal. Accordingly, we have
We have certain agreements with certain organizations with which we do business that contain indemnification provisions pursuant to which we typically agree to indemnify the party against certain types of third-party claims. We accrue for known indemnification issues when a loss is probable and can be reasonably estimated. There were
20
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
Our focus is to address the global smoking health and nicotine addiction epidemic through the development and commercialization of cytisinicline. Tobacco use is currently the leading cause of preventable death and is responsible for more than 8.0 million deaths worldwide and nearly half a million deaths in the United States annually. More than 87% of lung cancer deaths, 61% of all pulmonary disease deaths, and 32% of all deaths from coronary heart disease are attributable to smoking and exposure to secondhand smoke.
In addition, there are approximately 11.0 million adults in the United States who use e-cigarettes, also known as vaping. While nicotine e-cigarettes are thought to be less harmful than combustible cigarettes, they remain addictive and can deliver harmful chemicals which can cause lung injury or cardiovascular disease. In 2022, 2.55 million high school and middle school students reported using e-cigarettes. Research shows adolescents who have used e-cigarettes are seven times more likely to become smokers one year later compared to those who have never vaped. Currently, there are no U.S. Food and Drug Administration, or FDA, approved treatments indicated specifically as an aid to nicotine e-cigarette cessation.
Cytisinicline is a plant-based alkaloid with a high binding affinity to the nicotinic acetylcholine receptor. It is believed to aid in treating nicotine addiction for smoking and e-cigarette cessation by interacting with nicotine receptors in the brain, reducing the severity of withdrawal symptoms, and reducing the reward and satisfaction associated with nicotine products. Cytisinicline is an investigational product candidate being developed for treatment of nicotine addiction and has not been approved by the FDA for any indication in the United States.
We have no products approved for commercial sale and have not generated any revenue from product sales to date. We have never been profitable and have incurred operating losses in each year since inception. Our net loss was $9.0 million for the three months ended March 31, 2023. As of March 31, 2023, we had an accumulated deficit of $144.9 million, cash and cash equivalents balance of $16.5 million and a negative working capital balance of $2.1 million. For the three months ended March 31, 2023, net cash used in operating activities was $8.3 million.
Substantial doubt exists as to our ability to continue as a going concern. Our ability to continue as a going concern is subject to material uncertainty and dependent on our ability to obtain additional financing. We expect to incur significant expenses and increasing operating losses for at least the next several years as we continue our clinical development of, and seek regulatory approval for, cytisinicline and add personnel necessary to operate as a public company with an advanced clinical candidate. We expect that our operating losses will fluctuate significantly from quarter to quarter and year to year due to timing of clinical development programs and efforts to achieve regulatory approval. Without additional funds, we may be forced to delay, scale back or eliminate some of our research and development activities or other operations and potentially delay product development in an effort to provide sufficient funds to continue our operations. If any of these events occurs, our ability to achieve our development and commercialization goals would be adversely affected.
Our current resources are insufficient to fund our planned operations for the next twelve months. We will continue to require substantial additional capital to continue our clinical development activities. Accordingly, we will need to raise substantial additional capital to continue to fund our operations from the sale of our securities, debt, partnering arrangements, non-dilutive fundraising or other financing transactions in order to finance the remaining development and commercialization of our product candidate. The amount and timing of our future funding requirements will depend on many factors, including the pace and results of our clinical development efforts. The uncertainty with respect to our operations and the market generally due to increasing interest rates and inflation may also make it challenging to raise additional capital on favorable terms, if at all. Failure to raise capital as and when needed, on favorable terms or at all, will have a negative impact on our financial condition and our ability to develop our product candidate. In addition, current macroeconomic conditions have caused turmoil in the banking sector. For example, on March 10, 2023, Silicon Valley Bank, or SVB, one of our banking partners, was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation, or FDIC, as receiver. On March 26, 2023, it was announced that First-Citizens Bank & Trust Company would assume all of SVB’s deposits and loans as of March 27, 2023 and since that date we continue to have full access our cash and cash equivalents. Failure to raise capital as and when needed, on favorable terms or at all, will have a negative impact on our financial condition and our ability to develop our product candidate.
Our accompanying financial results have been prepared assuming we will continue to operate as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business. The financial results do not include any adjustments to the amounts and classification of assets and liabilities that might be necessary should we be unable to continue as a going concern. Such adjustments could be material.
21
Cytisinicline Ongoing and Recent Clinical Developments
Company-Sponsored Clinical Trials for Smoking Cessation Indication
Completed Phase 3 ORCA-2 Trial
In April 2022, we announced positive topline results for the Phase 3 ORCA-2 clinical trial. ORCA-2 was initiated in October 2020 and evaluated the efficacy and safety of 3 mg cytisinicline dosed three times daily compared to placebo in 810 adult smokers at 17 clinical sites in the United States. ORCA-2 participants were randomized to one of three study arms to determine the smoking cessation efficacy and safety profile of cytisinicline when administered for either 6 or 12 weeks, compared to placebo. All subjects received standard behavioral support and were assigned to one of the following groups:
The ORCA-2 study had two independent primary endpoints that evaluated the success of smoking abstinence for both 6-week and 12-week durations of cytisinicline treatment, compared to placebo. The primary endpoints for ORCA-2 were biochemically verified continuous abstinence measured during the last 4 weeks of each treatment duration. Both the 6- and 12-week cytisinicline treatments demonstrated significantly better quit rates than placebo with odds ratios, or ORs, of 8.0 and 6.3, respectively.
Subjects who received 6 weeks of cytisinicline treatment had 8.0 times higher odds, or likelihood, to have quit smoking during the last 4 weeks of treatment compared to subjects who received placebo (p<0.0001). The abstinence rate during weeks 3-6 was 25.3% for cytisinicline compared to 4.4% for placebo.
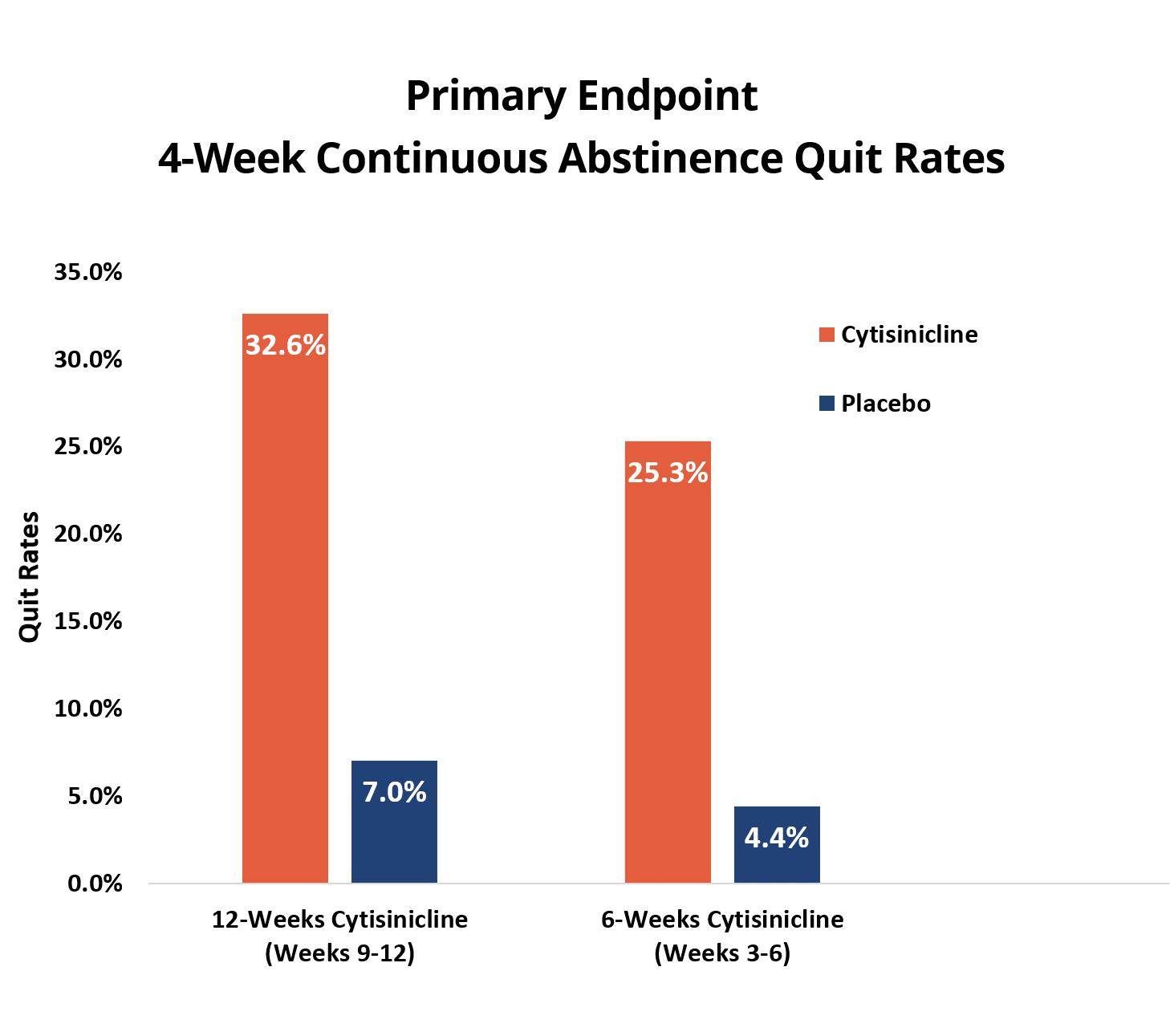
22
The secondary endpoints measured continuous abstinence after treatment out to 24 weeks. Both the 6- and 12-week secondary endpoints for continuous abstinence demonstrated significantly better quit rates for cytisinicline treated subjects than placebo. The continuous abstinence rate from week 9 to 24 was 21.1% for the 12-week cytisinicline arm compared to 4.8% for placebo, with an OR of 5.3 (p<0.0001). The continuous abstinence rate from week 3 to 24 was 8.9% for the 6-week cytisinicline arm compared to 2.6% for placebo, with an OR of 3.7 (p=0.0016).
A third secondary endpoint compared the two cytisinicline treatment arms and evaluated for an increased risk in relapse from week 6 to week 24 when subjects were switched to placebo during week 6 to week 12 (Arm B) instead of receiving cytisinicline for another 6 weeks during week 6 to week 12 (Arm C). The analysis showed that there was no increased risk of smoking relapse in subjects who had successfully quit smoking by week 3 through week 6 if they received placebo instead of continuing cytisinicline from week 6 to week 12.
Cytisinicline was well tolerated with no treatment-related serious adverse events reported. The most commonly reported adverse events (occurring greater than 5% overall in the study) for placebo, 6-week cytisinicline, and 12-week cytisinicline, respectively, were:
|
Placebo |
6-Weeks Cytisinicline |
12-Weeks Cytisinicline |
Insomnia |
4.8% |
8.6% |
9.6% |
Abnormal Dreams |
3.0% |
8.2% |
7.8% |
Headaches |
8.1% |
6.7% |
7.8% |
Nausea |
7.4% |
5.9% |
5.6% |
Additional analyses from the ORCA-2 trial were presented at the Society for Research on Nicotine and Tobacco, or SRNT, annual meeting in March 2023. We expect to submit additional findings for presentation at future medical conferences and are in the process of publishing the ORCA-2 trial results.
Ongoing Phase 3 ORCA-3 Trial
In January 2022, we initiated our Phase 3 ORCA-3 clinical trial. ORCA-3 is a confirmatory Phase 3 trial required for registrational approval of cytisinicline in the United States and has the same design as the Phase 3 ORCA-2 trial. The Phase 3 trial will evaluate the efficacy and safety of 3 mg cytisinicline dosed three times daily compared to placebo in 792 adult smokers at 20 clinical sites. ORCA-3 participants were randomized to one of three study arms to evaluate cytisinicline administered for either 6 or 12 weeks, compared to placebo. All subjects will receive standard behavioral support and will be assigned to one of the following groups:
The primary outcome measure of success in the ORCA-3 trial is biochemically verified continuous abstinence during the last four weeks of treatment in the 6 and 12-week cytisinicline treatment arms compared with placebo. Each treatment arm will be compared independently to the placebo arm, and the trial will be determined to be successful if either or both of the cytisinicline treatment arms show a statistical benefit compared to placebo. Secondary outcome measures will be conducted to assess continued abstinence rates through six months from the start of study treatment. Last subject last visit was completed in March of 2023, and we expect topline ORCA-3 data results to be reported in the second quarter of 2023.
Completed Phase 2 ORCA-1 Trial
In June 2019, we announced positive top line results for the Phase 2b ORCA-1 trial and defined the dose selection of 3 mg, three times daily, or TID, for our Phase 3 development. ORCA-1 was the first trial in our Ongoing Research of Cytisinicline for Addiction Program, or ORCA Program, that aims to evaluate the effectiveness of cytisinicline for smoking cessation, nicotine addiction therapy, and potential benefit in other indications.
23
ORCA-1 was initiated in October 2018 and evaluated 254 smokers in the United States. The trial evaluated both 1.5 mg and 3 mg doses of cytisinicline on the standard declining titration schedule as well as a more simplified TID dosing schedule, both over 25 days. The trial was randomized and blinded to compare the effectiveness of the cytisinicline doses and schedules to respective placebo groups. Subjects were treated for 25 days, provided behavioral support, and followed up for an additional four weeks to assess continued smoking abstinence after the 25-day treatment.
The primary endpoint in the study was the reduction in daily smoking, a self-reported measure. Three of the four cytisinicline treatment arms demonstrated a statistically significant reduction, p<0.05, compared to placebo. The fourth arm trended to significance (p= 0.052). Across all treatment arms, over the 25-day treatment period, subjects on cytisinicline experienced a 74-80% median reduction in the number of cigarettes smoked, compared to a 62% reduction in the placebo arms.
The secondary endpoint of the trial was a 4-week continuous abstinence rate, which is the relevant endpoint for regulatory approval. All cytisinicline treatment arms showed significant improvements in abstinence rates compared to the placebo arms. Notably, the 3 mg TID cytisinicline arm demonstrated a 50% abstinence rate at week 4, compared to 10% for placebo (p<0.0001) and a continuous abstinence rate, weeks 5 through 8, of 30% for cytisinicline compared to 8% for placebo (p= 0.005). Smokers in the 3 mg TID arm had an OR of 5.04 (95% CI: 1.42, 22.32) for continuous abstinence from week 5 to week 8, compared with placebo. In this study, the results indicated that smokers receiving 3 mg cytisinicline TID were five times more likely to stop smoking compared to smokers receiving placebo.
At week 4, all four cytisinicline arms demonstrated statistically significant (p<0.05) reductions in expired carbon monoxide, or CO, a biochemical measure of smoking activity. Expired CO levels had declined by a median of 71-80% in the cytisinicline treatment arms, compared to only 38% in the placebo arms. The greater reductions in expired CO levels for the cytisinicline arms versus placebo suggest that placebo-treated subjects may have over-reported their reduction in cigarettes smoked or overcompensated with greater inhalation while smoking fewer cigarettes.
Cytisinicline was well-tolerated with no serious adverse effects, or SAEs, reported. The most commonly reported (>5%) adverse effects, or AEs, across all cytisinicline treatment arms versus placebo arms were abnormal dreams, insomnia, upper respiratory tract infections, and nausea. In the 3 mg TID treatment arm versus placebo arms, the most common AEs were abnormal dreams, insomnia, and constipation (each 6% vs 2%), upper respiratory tract infections (6% vs 14%), and nausea (6% vs 10%), respectively. Compliance with study treatment was greater than 94% across all arms.
We presented the ORCA-1 results in September 2019 at the annual European meeting of the SRNT, held in Oslo, Norway and the trial results were published in the journal Nicotine and Tobacco Research in April 2021. Based on the results of the ORCA-1 trial, we have selected 3 mg TID for Phase 3 development. Overall, the 3 mg dose administered TID demonstrated the best overall safety and efficacy when compared to the 1.5 mg dose or the declining titration schedule evaluated in ORCA-1. At the SRNT European meeting held in September 2021, exploratory analyses were presented that showed cytisinicline treatment had an earlier onset of sustained abstinence compared to placebo and that the cytisinicline TID schedule appeared more effective for achieving sustained abstinence in smokers who had previously failed to quit on varenicline compared to the declining titration schedule.
In November 2019, we held a type C meeting with the U.S. Food and Drug Administration, or FDA, to review the ORCA-1 results and our revisions to the Phase 3 clinical program using the simplified 3 mg TID dosing schedule. The FDA agreed that the 3 mg TID dosing schedule was acceptable.
Other Clinical Trials
We are currently conducting two clinical studies required for the NDA: one pharmacokinetics, or PK, study to evaluate for any increased cytisinicline blood levels in subjects who have various levels of renal impairment, and another study to evaluate for any effects of cytisinicline on QT interval prolongation. Plans for both studies, were first discussed with the FDA as part of an end of Phase 2 meeting in 2018, followed by more detailed review and agreement with the FDA during 2022. In addition, we are planning to conduct a final PK study to determine various remaining PK parameters for the 3 mg TID cytisinicline regimen, including the timing of steady state dosing. Results from these studies are anticipated to be available by the end of 2023.
Company-Sponsored Clinical Trials for an E-cigarette (nicotine vaping) Cessation Indication
Completed Phase 2 ORCA-V1 Clinical Trial
In July 2021, we announced that we were awarded a grant from the National Institute on Drug Abuse, or NIDA, of the National Institutes of Health, or NIH, to evaluate the use of cytisinicline as a treatment for cessation of nicotine e-cigarette use. This initial grant award, in the amount of $320,000, disbursed on August 1, 2021, and was utilized to complete critical regulatory and clinical operational activities, such as protocol finalization, clinical trial site identification, and submission of an Investigational New Drug Application, or IND, to the FDA for investigating cytisinicline in nicotine e-cigarette users. In November 2021, we announced that the
24
FDA had completed their review and accepted the IND application to investigate cytisinicline as a cessation treatment in this population.
In June 2022, following NIDA/NIH review of completed milestones, we announced that we were awarded the next grant funding from NIDA in the amount of approximately $2.5 million. The full grant award of $2.8 million is expected to cover approximately half of the total ORCA-V1 clinical study costs. The Primary Investigators for the grant are our President and Chief Medical Officer, Dr. Cindy Jacobs, and Dr. Nancy Rigotti, Professor of Medicine at Harvard Medical School and Director, Tobacco Research and Treatment Center, Massachusetts General Hospital.
In June 2022, we announced the initiation of the Phase 2 ORCA-V1 clinical trial. In April 2023, we reported positive topline results showing a statistically significant vaping cessation benefit for cytisinicline-treated participants in the ORCA-V1 trial.
ORCA-V1 evaluated 160 adults who used e-cigarettes on a daily basis at five clinical trial locations in the United States. ORCA-V1 participants were randomized to receive 3 mg cytisinicline three times daily or placebo for 12 weeks in combination with standard cessation behavioral support.
The primary endpoint for ORCA-V1 was biochemically verified continuous abstinence from nicotine e-cigarette use, measured during the last 4 weeks of treatment. Subjects who received 12 weeks of cytisinicline treatment had 2.6 times higher odds, or likelihood, to have quit vaping during the last four weeks of treatment compared to subjects who received placebo (p=0.035). The vaping cessation rate during weeks 9 through 12 was 31.8% for cytisinicline compared to 15.1% for placebo. A benefit in favor of cytisinicline was consistently observed across the secondary endpoints. Additionally, a cessation benefit was observed for cytisinicline across clinical trial sites and participant demographics such as age, gender, race, or whether they had smoked cigarettes in the past.
Cytisinicline was well tolerated and no serious adverse events, or AEs, were reported. Similar rates of AEs were observed between treatment arms (54.7% in the placebo arm vs. 50.9% in the cytisinicline arm). The most commonly reported (>5%) AEs in the placebo arm, in order of frequency, were nausea, COVID-19 infection, headache, anxiety, and upper respiratory tract infection. In the cytisinicline arm, >5% AEs reported, in order of frequency, were sleep disturbances, anxiety, headache, fatigue, and upper respiratory tract infection.
Other Recent Investigator-Sponsored Clinical Trials
In June 2020, we announced the topline results from the independent, investigator-sponsored Phase 3 RAUORA trial. RAUORA was a non-inferiority study comparing cytisinicline to Chantix (varenicline) in Māori (indigenous New Zealanders) and whānau (family) of Māori. The study was led by Dr. Natalie Walker, Associate Professor at the University of Auckland, and was funded by the Health Research Council of New Zealand. The study enrollment was planned for 2,140 subjects. In total, 1,105 Māori or whānau expressed interest in participating in the study and a total of 679 were randomized to receive either cytisinicline or varenicline. The average age of participants in the trial was 43 years and approximately 70% of the participants were women.
The study compared cytisinicline administered on a schedule of 25 days of declining titration followed by twice-daily dosing for a total of 12 weeks with varenicline administered on a schedule of seven days of inclining titration followed by twice-daily dosing for a total of 12 weeks. The primary endpoint was a comparison of biochemically confirmed continuous abstinence rates at six months, and the trial was designed to assess if the two agents were non-inferior to each other.
The primary endpoint of the non-inferiority trial was to demonstrate that cytisinicline quit rates would be no less than 10% lower than the quit rates for varenicline. Topline results indicated that the RAUORA trial achieved its primary endpoint in showing that cytisinicline plus behavioral support was at least as effective as varenicline plus behavioral support at six months. Cytisinicline met the pre-specified non-inferiority endpoint and was trending towards superiority with an Absolute Risk Difference of +4.29 in favor of cytisinicline (95% CI -0.22 to 8.79), demonstrating a 4.29% improvement in quit rates in favor of cytisinicline. Specifically, continuous abstinence rates at six months, verified by expired CO, were 12.1% for cytisinicline compared to 7.9% for varenicline. The Relative Risk was 1.55 on an intent-to-treat basis, indicating that subjects in the cytisinicline arm were approximately one and a half times more likely to have quit smoking at six months compared to subjects who received varenicline.
Additionally, significantly fewer overall AEs were reported in cytisinicline-treated subjects (Relative Risk 0.56, 95% CI 0.49 to 0.65, p<0.001). Notably, of the subjects who experienced adverse events, cytisinicline subjects reported significantly less nausea, insomnia and vivid dreams (p<0.05).
The final RAUORA trial results and additional analyses were presented at the SRNT European Annual Meeting in September 2020 and were published in the journal Addiction in March 2021. Also presented at the SRNT Europe Annual Meeting in September 2020 were results from a preclinical study conducted at the University of Cambridge Department of Biochemistry. The study was designed to examine the in vitro binding characteristics of cytisinicline compared to varenicline at the human 5-HT3 receptor. Using a
25
radioligand antagonist displacement design, the study reported an IC50 of 0.50 mM for cytisinicline and 0.25 µM for varenicline, representing a 2000-greater fold agonist binding affinity to the 5-HT3 receptor for varenicline compared to cytisinicline. Agonist activation of 5-HT3 receptors in the brain stem has been shown to induce nausea and vomiting. The data demonstrating the difference in binding potency at the 5-HT3 receptor provide potential rationale for the lower overall incidence of adverse events reported for cytisinicline compared to varenicline.
Non-clinical Studies
Non-clinical toxicology studies were sponsored by the National Center for Complementary and Integrative Health, or NCCIH, a division of the NIH and by the National Cancer Institute, or NCI, to assist in our IND for investigating cytisinicline as a smoking cessation treatment. We filed this IND application for cytisinicline with the FDA in 2017, which included the NCCIH sponsored non-clinical studies. Additional NCCIH and NCI sponsored non-clinical toxicology studies were later submitted in support for initiating our Phase 3 program.
Non-clinical toxicology studies that are required for a New Drug Application, or NDA, include two longer-term chronic toxicology studies and two carcinogenicity studies, which were completed as company-sponsored studies and submitted to the FDA.
License & Supply Agreements
Sopharma License and Supply Agreements
We are party to a license agreement, or the Sopharma License Agreement, and a supply agreement, or the Sopharma Supply Agreement, with Sopharma. Pursuant to the Sopharma License Agreement, we were granted access to all available manufacturing, efficacy and safety data related to cytisinicline, as well as a granted patent in several European countries related to new oral dosage forms of cytisinicline providing enhanced stability. Additional rights granted under the Sopharma License Agreement include the exclusive use of, and the right to sublicense, certain cytisinicline trademarks in all territories described in the Sopharma License Agreement. Under the Sopharma License Agreement, we agreed to pay a nonrefundable license fee. In addition, we agreed to make certain royalty payments equal to a mid-single digit percentage of all net sales of cytisinicline products in our territory during the term of the Sopharma License Agreement, including those sold by a third party pursuant to any sublicense which may be granted by us. To date, any amounts paid to Sopharma pursuant to the Sopharma License Agreement have been immaterial.
Research and Development Expenses
Research and development, or R&D, expenses consist primarily of costs for clinical trials, contract manufacturing, personnel costs, milestone payments to third parties, facilities, regulatory activities, non-clinical studies and allocations of other R&D-related costs. External expenses for clinical trials include fees paid to clinical research organizations, clinical trial site costs and patient treatment costs.
We manage our clinical trials through contract research organizations and independent medical investigators at our sites and at hospitals and expect this practice to continue. Due to our ability to utilize resources across several projects, we do not record or maintain information regarding the indirect operating costs incurred for our R&D programs on a program-specific basis. In addition, we believe that allocating costs on the basis of time incurred by our employees does not accurately reflect the actual costs of a project.
We expect our R&D expenses to increase for the foreseeable future as we continue to conduct our ongoing non-clinical studies, and initiate new clinical trials and registration-enabling activities. The process of conducting clinical trials and non-clinical studies necessary to obtain regulatory approval is costly and time consuming and we may never succeed in achieving marketing approval for cytisinicline. (See “Item 1A. Risk Factors—Risks Related to the Development of Our Product Candidate Cytisinicline.”)
Successful development of cytisinicline is highly uncertain and may not result in an approved product. We cannot estimate completion dates for development activities or when we might receive material net cash inflows from our R&D projects, if ever. We anticipate we will make determinations as to which markets, and therefore, which regulatory approvals, to pursue and how much funding to direct toward achieving regulatory approval in each market on an ongoing basis in response to our ability to enter into new strategic alliances with respect to each program or potential product candidate, the scientific and clinical success of each future product candidate, and ongoing assessments as to each future product candidate’s commercial potential. We will need to raise additional capital and may seek additional strategic alliances in the future in order to advance our various programs.
Our projects or intended R&D activities may be subject to change from time to time as we evaluate results from completed studies, our R&D priorities and available resources.
26
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for our personnel in executive, finance and accounting, corporate communications and other administrative functions, as well as consulting costs, including market research, business consulting, human resources and intellectual property. Other costs include professional fees for legal and auditing services, insurance and facility costs.
Results of Operations
For the three months ended March 31, 2023 and 2022
Research and development expenses
Our R&D expenses for our clinical development program for the three months ended March 31, 2023 and 2022 were as follows (in thousands):
|
|
Three Months Ended |
|
|
|||||
|
|
March 31, |
|
|
|||||
|
|
2023 |
|
|
2022 |
|
|
||
Clinical development programs: |
|
|
|
|
|
|
|
||
Cytisinicline |
|
$ |
5,534 |
|
|
$ |
4,388 |
|
|
Total research and development expenses |
|
$ |
5,534 |
|
|
$ |
4,388 |
|
|
R&D expenses for the three months ended March 31, 2023 increased to $5.5 million from $4.4 million for the three months ended March 31, 2022. The increase in the three months ended March 31, 2023 as compared to the same periods in 2022 was primarily due to higher costs from our Phase 3 ORCA-3 trial and Phase 2 ORCA-V1 trial as both continued fully enrolled during the quarter and had their last subject last visit completed in March 2023, as compared to the same period in 2022 where our Phase 3 ORCA-2 trial had its last subject last visit completed in January 2022.
General and administrative expenses
Our general and administrative expenses for the three months ended March 31, 2023 and 2022 were as follows (in thousands):
|
|
Three Months Ended |
|
|
|||||
|
|
March 31, |
|
|
|||||
|
|
2023 |
|
|
2022 |
|
|
||
Total general and administrative expenses |
|
$ |
3,044 |
|
|
$ |
2,838 |
|
|
General and administrative expenses for the three months ended March 31, 2023 increased to $3.0 million from $2.8 million for the three months ended March 31, 2022. The increase was primarily due to higher employee expenses associated with stock-based compensation and higher legal expenses as a result of general corporate activities. The increased expenses were partially offset by a decrease in costs associated with clinical trial media and awareness as both the ORCA-3 trial and ORCA-V1 trial were fully enrolled in the third and fourth quarter of 2022, respectively.
Total Other Income (Expenses)
Total other expenses for the three months ended March 31, 2023 and 2022 were $0.4 million and $0.3 million, respectively. The increase in 2023 as compared to 2022 was due mainly to higher interest expense on our convertible debt, this was partially offset by higher interest income in 2023 as a result of higher interest rates
27
Liquidity and Capital Resources
We have incurred an accumulated deficit of $144.9 million through March 31, 2023 and we expect to incur substantial additional losses in the future as we operate our business and continue or expand our R&D activities and other operations. We have not generated any revenue from product sales to date, and we may not generate product sales revenue in the near future, if ever. As of March 31, 2023, we had a cash and cash equivalents balance of $16.5 million and a negative working capital balance of $2.1 million. We believe that our existing cash and cash equivalents will be sufficient for us to fund our current operating expenses and capital expenditures into late 2023.
The financial results have been prepared assuming we will continue to operate as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business.
Substantial doubt exists as to our ability to continue as a going concern. Our ability to continue as a going concern is subject to material uncertainty and dependent on our ability to obtain additional financing. We have historically financed our operations through equity and debt financings. There can be no assurance that financing from these or other sources will be available to us in the future. Without additional funds, we may be forced to delay, scale back or eliminate some of our research and development activities or other operations and potentially delay product development in an effort to provide sufficient funds to continue our operations. If any of these events occur, our ability to achieve our development and commercialization goals would be adversely affected.
Our current resources are insufficient to fund our planned operations for the next twelve months. We will continue to require substantial additional capital to continue our clinical development activities. Accordingly, we will need to raise substantial additional capital to continue to fund our operations from the sale of our securities, debt, partnering arrangements, non-dilutive fundraising or other financing transactions in order to finance the remaining development and commercialization of our product candidate. The amount and timing of our future funding requirements will depend on many factors, including the pace and results of our clinical development efforts. The uncertainty with respect to our operations and the market generally due to increasing interest rates and inflation may also make it challenging to raise additional capital on favorable terms, if at all. In addition, current macroeconomic conditions have caused turmoil in the banking sector. For example, on March 10, 2023, Silicon Valley Bank, or SVB, one of our banking partners, was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation, or FDIC, as receiver. On March 26, 2023, it was announced that First-Citizens Bank & Trust Company would assume all of SVB’s deposits and loans as of March 27, 2023. Failure to raise capital as and when needed, on favorable terms or at all, will have a negative impact on our financial condition and our ability to develop our product candidate.
In addition, we expect to incur significant expenses and increasing operating losses for at least the next several years as we continue our clinical development of, seek regulatory approval for, and commercialize, cytisinicline and add personnel necessary to operate as a commercial-stage public company. We expect that our operating losses will fluctuate significantly from quarter to quarter and year to year due to timing of clinical development programs and efforts to achieve regulatory approval.
The consolidated financial results do not include any adjustments to the amounts and classification of assets and liabilities that might be necessary should we be unable to continue as a going concern. Such adjustments could be material.
We did not have during the periods presented, and we do not currently have, any commitments or obligations, including contingent obligations, arising from arrangements with unconsolidated entities or persons that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, cash requirements or capital resources.
Convertible Debt and Term Loan
On December 22, 2021, we entered into a $25.0 million contingent convertible debt agreement, or Original Debt Agreement, with Silicon Valley Bank, or SVB, and SVB Innovation Credit Fund VIII, L.P., or, together with SVB, the Lenders. As part of the Original Debt Agreement, the Lenders funded $15.0 million in the form of convertible indebtedness, or Convertible Debt, at closing. On April 26, 2022, we entered into (i) a loan and security agreement, or Loan Agreement, with SVB for the remaining $10.0 million remaining in the Original Debt Agreement, pursuant to which SVB provided a commitment to extend term loans having an aggregate original principal amount of up to $10.0 million, or Term Loans, and (ii) a first amendment to the Original Debt Agreement, or the Amendment, and as amended by the Amendment, the Debt Agreement.
Under the terms of the agreement, the Convertible Debt matures on December 22, 2023 and may be extended to December 22, 2024 upon our written request and SVB’s approval on or prior to December 22, 2023. The Convertible Debt will accrue interest at the aggregate of (a) a floating rate per annum equal to the greater of (i) 2.25% and (ii) the prime rate minus 1.0%, which interest is payable in cash monthly in arrears, and (b) 7.0% per annum, which interest shall compound monthly.
28
Subject to certain terms and conditions, the Lenders may convert all or any part of the outstanding Convertible Debt and accrued and unpaid interest at any time prior to maturity into shares of our common stock at a conversion price equal to $9.34 per share, subject to customary anti-dilution adjustments. Additionally, all outstanding Convertible Debt, including accrued and unpaid interest, will mandatorily convert into shares of our common stock, at the conversion price, on such date, if any, when the closing price per share of our common stock has been equal to or greater than $24.00 for thirty consecutive trading days prior to such date.
We have the right, or Call Right, at any time to repay and retire all (but not less than all) of the outstanding Convertible Debt and accrued and unpaid interest, if any, prior to its conversion by payment of a premium determined based on the date of such repayment equal to:
in either case together with all accrued and unpaid interest on the principal balance of the Convertible Debt. If the Call Right is exercised by us, the Lenders will retain certain lookback rights in the event we enter into an agreement to be acquired in the twelve months following the exercise of the Call Right. We agreed to grant the Lenders a security interest in virtually all of our assets, including our patents and other intellectual property as security for our obligations under the Debt Agreement.
Subject to the terms and conditions of the Loan Agreement, we may borrow term loans under the Loan Agreement until April 30, 2023. Amounts borrowed under the Loan Agreement will incur interest at a floating rate equal to the greater of 3.50% and the Wall Street Journal, or WSJ, prime rate, and will be subject to interest only payments through April 30, 2024. Commencing on May 1, 2024, the outstanding loans under the Loan Agreement will be repaid in 24 consecutive equal monthly installments of principal plus accrued and unpaid interest. The Term Loans mature on April 1, 2026. Upon the earliest to occur of the maturity date, repayment of the Term Loans in full, acceleration of the loans or termination of the Loan Agreement, we will be required to pay a final payment equal to the aggregate principal amount of the Term Loan advances extended by SVB multiplied by 6.0%. Our obligations under the Loan Agreement are secured by substantially all of our assets, other than our intellectual property.
Upon and after borrowing under the Loan Agreement, we must comply with certain financial covenants as set forth in the Loan Agreement and the Amendment, including a minimum liquidity ratio of at least 1.25 to 1.00, or at our election after receiving at least $30 million in net cash proceeds from the issuance and sale of equity securities, a minimum market capitalization of at least $250 million. The Loan Agreement also contains customary affirmative and restrictive covenants, including covenants regarding the incurrence of additional indebtedness or liens, investments, transactions with affiliates, delivery of financial statements, payment of taxes, maintenance of insurance, dispositions of property, mergers or acquisitions, among other customary covenants. We are also restricted from paying dividends or making other distributions or payments on its capital stock, subject to limited exceptions. The Loan Agreement includes customary representations and warranties, events of default and termination provisions. In addition to the financial covenants described above, the Amendment makes certain other changes to the Original Debt Agreement related to our entry into the Loan Agreement. The availability of Term Loans under the Loan Agreement expired on April 30, 2023, with no amounts drawn under the facility.
At-the-Market Sales Agreement
On December 21, 2021, we entered into an At-the-Market Offering Sales Agreement, or ATM, with Virtu Americas, LLC, as sales agent, pursuant to which we may sell shares of common stock with an aggregate offering price of up to $25 million.
Since entry into the ATM, from December 21, 2021 through March 31, 2023, we offered and sold an aggregate of 200,000 shares of our common stock. These aggregate sales resulted in gross proceeds to us of approximately $1.5 million. As of March 31, 2023, shares of our common stock having an aggregate value of approximately $23.5 million remained available for sale under the ATM.
During the three months ended March 31, 2023, we did not sell any shares of our common stock pursuant to the ATM.
November 2022 Private Placement
In November 2022, we entered into subscription agreements with certain accredited investors pursuant to which we sold to the purchasers in a private placement transaction approximately 4,093,141 units at a purchase price of $4.625 per unit, with each unit consisting of two shares of common stock and a common stock purchase warrant to purchase one share of common stock, or the November 2022 Warrants.
29
The November 2022 Warrants are exercisable at a price per share of common stock of $4.50, subject to adjustment. The November 2022 Warrants are exercisable beginning on the six-month anniversary of the initial closing date of the private placement offering, May 18, 2023, or the Initial Exercise Date, and will expire on the seven year anniversary of the initial closing date of the private placement offering, or November 18, 2029. The November 2022 Warrants cannot be exercised by a warrant holder if, after giving effect thereto, such warrant holder would beneficially own more than 19.99% of our outstanding common stock. Additionally, subject to certain exceptions, if, after the Initial Exercise Date, (i) the volume weighted average price of our common stock for each of 30 consecutive trading days, or the November 2022 Measurement Period, which November 2022 Measurement Period commenced on November 18, 2022, exceeds 300% of the exercise price (subject to adjustments for stock splits, recapitalizations, stock dividends and similar transactions), (ii) the average daily trading volume for such November 2022 Measurement Period exceeds $500,000 per trading day and (iii) certain other equity conditions are met, and subject to a beneficial ownership limitation, then we may call for cancellation of all or any portion of the November 2022 Warrants then outstanding.
We received approximately $17.8 million in net proceeds from the private placement after deducting placement agent expenses and commissions and offering expenses.
Cash Flows
Cash Used in Operating Activities
For the three months ended March 31, 2023, net cash used in operating activities was $8.3 million compared to $6.8 million for the three months ended March 31, 2022. The increase in cash used in operations in the 2023 period as compared to the 2022 period was primarily due to higher costs from our Phase 3 ORCA-3 trial and Phase 2 ORCA-V1 trial as both continued fully enrolled during the quarter and had their last subject last visit completed in March 2023, as compared to the same period in 2022 where our Phase 3 ORCA-2 trial had its last subject last visit completed in January 2022.
Cash Provided in Financing Activities
For the three months ended March 31, 2023, net cash provided by financing activities was $47,000 compared to $95,000 for the three months ended March 31, 2022. Net cash provided by financing activities in the three months ended March 31, 2023 relates to proceeds received from warrant exercises. Net cash provided by financing activities in the three months ended March 31, 2022, relates to proceeds received from ATM sales and warrant exercises.
Commitments and Contingencies
We previously disclosed certain contractual obligations and contingencies and commitments relevant to us within the financial statements and Management’s Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the SEC on March 16, 2023. There have been no material changes to our “Contractual Obligations” table in Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our 2022 Form 10-K. For more information regarding our current contingencies and commitments, see Note 9 to the financial statements included above.
Critical Accounting Policies and Estimates
The preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect reported amounts and related disclosures. We have discussed those estimates that we believe are critical and require the use of complex judgment in their application in our audited financial statements for the year ended December 31, 2022 in our Annual Report on Form 10-K filed with the SEC, on March 16, 2023. Since December 31, 2022, there have been no material changes to our critical accounting policies or the methodologies or assumptions we apply under them.
New Accounting Standards
See Note 2, “Accounting Policies,” of the consolidated financial statements for information related to the adoption of new accounting standards in 2023, no new accounting standards were adopted.
30
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that material information required to be disclosed in our periodic reports filed or submitted under the Securities Exchange Act of 1934, as amended, or the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Our disclosure controls and procedures are also designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act are accumulated and communicated to our management, including our principal executive officer and principal financial officer as appropriate, to allow timely decisions regarding required disclosure.
During the quarter ended March 31, 2023, we carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Based upon that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective, as of the end of the period covered by this report.
Changes in Internal Control Over Financial Reporting
We have not made any changes to our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act) during the quarter ended March 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls
Our management does not expect that our disclosure controls and procedures or our internal controls will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected.
31
PART II. OTHER INFORMATION
ITEM 1A. RISK FACTORS
Investing in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information contained in this Quarterly Report on Form 10-Q and in the other periodic and current reports and other documents we file with the Securities and Exchange Commission, before deciding to invest in our common stock. If any of the following risks materialize, our business, financial condition, results of operation and future prospects will likely be materially and adversely affected. In that event, the market price of our common stock could decline and you could lose all or part of your investment. This list is not exhaustive and the order of presentation does not reflect management's determination of priority or likelihood.
Risks Related to Our Financial Condition and Capital Requirements
Substantial doubt exists as to our ability to continue as a going concern. Our ability to continue as a going concern is subject to material uncertainty and dependent on our success at raising additional capital sufficient to meet our obligations on a timely basis. If we fail to obtain additional financing when needed, we may be unable to complete the development, regulatory approval and commercialization of our product candidate.
Substantial doubt exists as to our ability to continue as a going concern. Our ability to continue as a going concern is subject to material uncertainty and dependent on our ability to obtain additional financing. There is no assurance that we will obtain financing from other sources. The uncertainty with respect to our operations and the capital markets generally may make it more challenging to raise additional capital on favorable terms, if at all.
In addition, we expect to incur significant expenses and increasing operating losses for at least the next several years as we continue our clinical development of, seek regulatory approval for, and commercialize, cytisinicline and add personnel necessary to operate as a commercial-stage public company. We expect that our operating losses will fluctuate significantly from quarter to quarter and year to year due to timing of clinical development programs and efforts to achieve regulatory approval.
Our current resources are insufficient to fund our planned operations for the next twelve months. We will continue to require substantial additional capital to continue our clinical development activities. Accordingly, we will need to raise substantial additional capital to continue to fund our operations from the sale of our securities, debt, partnering arrangements, non-dilutive fundraising or other financing transactions in order to finance the remaining development and commercialization of our product candidate. The current financing environment in the United States, particularly for biotechnology companies like us, is exceptionally challenging and we can provide no assurances as to when such environment will improve. Our business may be impacted by macroeconomic conditions, including inflation, rising interest rates and volatile market conditions as well as political events, war, terrorism, business interruptions and other geopolitical events and uncertainties beyond our control. Further, the uncertainty with respect to our operations and the market generally due to increasing interest rates and inflation may also make it challenging to raise additional capital on favorable terms, if at all. Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. A severe or prolonged economic downturn could result in a variety of risks to our business, including in our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy also could strain our suppliers, possibly resulting in supply disruption. In addition, current macroeconomic conditions have caused turmoil in the banking sector. For example, on March 10, 2023, Silicon Valley Bank, or SVB, one of our banking partners, was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation, or FDIC, as receiver. On March 26, 2023, it was announced that First-Citizens Bank & Trust Company, or First Citizens, would assume all of SVB’s deposits and loans as of March 27, 2023. Under the terms of our Convertible Debt Agreement, we are required to keep substantially all of our cash and investments with SVB. While we were afforded full access to our cash and cash equivalents with SVB on March 13, 2023, we may be impacted by other disruptions to the U.S. banking system caused by the recent developments involving SVB, including potential delays in our ability to transfer funds whether held with SVB or otherwise and in the short-term potential delays in making payments to vendors while new banking relationships are established. For these reasons, among others, we cannot be certain that additional financing will be available when and as needed or, if available, that it will be available on acceptable terms. If financing is available, it may be on terms that adversely affect the interests of our existing stockholders. If adequate financing is not available, we may need to continue to reduce or eliminate our expenditures for research and development of cytisinicline, and may be required to suspend development of cytisinicline. Our actual capital requirements will depend on numerous factors, including:
32
We may not be able to secure sufficient financing on acceptable terms, or at all. Without additional funds, we may be forced to delay, scale back or eliminate some of our research and development activities or other operations and potentially delay product development in an effort to provide sufficient funds to continue our operations. If any of these events occur, our ability to achieve our development and commercialization goals would be adversely affected.
We have incurred substantial debt, which could impair our flexibility and access to capital and adversely affect our financial position, and our business would be materially adversely affected if we are unable to service our debt obligations.
On December 22, 2021, we entered into a $25.0 million contingent convertible debt agreement, or Original Debt Agreement, with Silicon Valley Bank, or SVB, and SVB Innovation Credit Fund VIII, L.P., or, together with SVB, the Lenders. As part of the Original Debt Agreement, the Lenders funded $15.0 million in the form of convertible indebtedness, or Convertible Debt, at closing. On April 26, 2022, we entered into (i) a loan and security agreement, or Loan Agreement, with SVB for the $10.0 million remaining in the Original Debt Agreement, pursuant to which SVB provided a commitment to extend term loans having an aggregate original principal amount of up to $10.0 million, or Term Loans, and (ii) a first amendment to the Original Debt Agreement, or the Amendment, and as amended by the Amendment, the Debt Agreement. As of March 31, 2023, the principal amounts due under our debt instruments (including the Loan Agreement and Debt Agreement) totaled $16.4 million, of which $16.4 million is classified as current.
Under the terms of the Debt Agreement, the Convertible Debt matures on December 22, 2023 and may be extended to December 22, 2024 upon our written request and SVB’s approval on or prior to December 22, 2023. The Convertible Debt will accrue interest at the aggregate of (a) a floating rate per annum equal to the greater of (i) 2.25% and (ii) the prime rate minus 1.0%, which interest is payable in cash monthly in arrears, and (b) 7.0% per annum, which interest shall compound monthly. Subject to the terms and conditions of the Loan Agreement, we may borrow term loans under the Loan Agreement until April 30, 2023. Amounts borrowed under the Loan Agreement will incur interest at a floating rate equal to the greater of 3.50% and the Wall Street Journal prime rate, and will be subject to interest only payments through April 30, 2024. Commencing on May 1, 2024, the outstanding loans under the Loan Agreement will be repaid in 24 consecutive equal monthly installments of principal plus accrued and unpaid interest. The Term Loans mature on April 1, 2026. Upon the earliest to occur of the maturity date, repayment of the Term Loans in full, acceleration of the loans or termination of the Loan Agreement, we will be required to pay a final payment equal to the aggregate principal amount of the Term Loan advances extended by SVB multiplied by 6.0%. Our obligations under the Loan Agreement are secured by substantially all of our assets, other than our intellectual property. The availability of Term Loans under the Loan Agreement expired on April 30, 2023, with no amounts drawn under the facility.
Our indebtedness may:
33
Servicing our debt requires a significant amount of cash. Our debt is subject to floating interest rates set in relation to the prime rate. Increases in interest rates have, and may continue, to make our debt service costs increase. Additionally, we currently do not generate any cash flow from operations, and if we are unable to pay our debt service costs or repay it when it becomes due, we would be in default under our Loan and Security Agreement and Debt Agreement. We may be required to raise additional working capital through future financings or sales of assets to enable us to repay our outstanding indebtedness as it becomes due. There can be no assurance that we will be able to generate cash or raise additional capital. If we are at any time unable to service our indebtedness when payment is due, we may be required to attempt to renegotiate the terms of the instruments relating to the indebtedness, seek to refinance all or a portion of the indebtedness or obtain additional financing. There can be no assurance that we would be able to successfully renegotiate such terms, that any such refinancing would be possible or that any additional financing could be obtained on terms that are favorable or acceptable to us, if at all. Any debt financing that is available could cause us to incur substantial costs and subject us to covenants that significantly restrict our ability to conduct our business. If we seek to complete additional equity financings, the interests of existing stockholders may be diluted. If we are unable to make payment on our secured debt instruments when due, the lender under such instrument may foreclose on and sell the assets securing such indebtedness to satisfy our payment obligations, which could prevent us from accessing those assets for our business and conducting our business as planned, which could materially harm our financial condition and results of operations.
Further, the Loan Agreement contains customary affirmative and restrictive covenants, including covenants regarding the incurrence of additional indebtedness or liens, investments, transactions with affiliates, delivery of financial statements, payment of taxes, maintenance of insurance, dispositions of property, mergers or acquisitions, and the requirement we keep substantially all of our cash and investments with SVB, among other customary covenants. We are also restricted from paying dividends or making other distributions or payments on its capital stock, subject to limited exceptions. The Loan Agreement includes customary representations and warranties, events of default and termination provisions. We must also comply with certain financial covenants as set forth in the Loan Agreement and the Amendment if we draw down on the $10.0 million Term Loans available, including a minimum liquidity ratio of at least 1.25 to 1.00, or at our election after receiving at least $30 million in net cash proceeds from the issuance and sale of equity securities, a minimum market capitalization of at least $250 million. As of March 31, 2023 no amounts had been drawn on the Term Loans. The availability of Term Loans under the Loan Agreement expired on April 30, 2023, with no amounts drawn under the facility.
If we default under the Loan Agreement, the Lenders will be able to declare all obligations immediately due and payable and take control of our collateral, potentially requiring us to renegotiate our agreement on terms less favorable to us or to immediately cease operations. Further, if we are liquidated, the rights of the Lenders to repayment would be senior to the rights of the holders of our common stock to receive any proceeds from the liquidation. The Lenders could declare a default under the Loan Agreement upon the occurrence of any event that the Lenders interpret as a material adverse change as defined under the Loan Agreement, thereby requiring us to repay the loan immediately or to attempt to reverse the declaration of default through negotiation or litigation. Any declaration by the Lenders of an event of default could significantly harm our business, financial condition, results of operations and prospects and could cause the price of our common stock to decline. If we raise any additional debt financing, the terms of such additional debt could further restrict our operating and financial flexibility.
With the closure of SVB and appointment of the Federal Deposit Insurance Corporation, or the FDIC, as receiver on March 10, 2023, and subsequent assumption by First Citizens all of SVB’s deposits and loans, we are aware that there can be no assurance that SVB, First Citizens or any other successor lender(s) will be willing to work with us on any modifications to the current Convertible Debt agreement. Any amounts due under the Convertible Debt, including interest payments, will remain payable to any successor lender(s).
We have incurred losses since inception, have a limited operating history on which to assess our business and anticipate that we will continue to incur losses for the foreseeable future.
We are a clinical development-stage specialty pharmaceutical company with a limited operating history, are not profitable, have incurred losses in each year since our inception and expect to continue incurring losses for the foreseeable future.
Pharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We have devoted substantially all of our financial resources to identify, acquire, and develop cytisinicline, including providing general and administrative support for our operations. To date, we have financed our operations primarily through the sale of equity securities and
34
convertible promissory notes. The amount of our future net losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity or debt financings, strategic collaborations, or grants.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We further expect that our expenses will increase substantially if and as we:
Further, the net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance.
We have never generated any revenue from product sales and may never be profitable.
We have no products approved for commercialization and have never generated any revenue from product sales. Our ability to generate revenue and achieve profitability depends on our ability, alone or with strategic collaborators, to successfully complete the development of, and obtain the regulatory and marketing approvals necessary to commercialize cytisinicline. We do not anticipate generating revenue from product sales for the foreseeable future. Our ability to generate future revenue from product sales depends heavily on our success in many areas, including but not limited to:
35
Even if a product candidate that we develop is approved for commercial sale, we anticipate incurring significant costs associated with commercializing that candidate. Additionally, if we are not able to generate sufficient revenue from the sale of any approved products to cover our operating costs, we may never become profitable. If we obtain regulatory approval to market a product candidate, our future revenue will depend upon the size of any markets in which our product candidate may receive approval, and our ability to achieve sufficient market acceptance, pricing, reimbursement from third-party payors, and adequate market share for our product candidate in those markets.
Our cash and cash equivalents could be adversely affected if the financial institutions in which we hold our cash and cash equivalents fail.
We regularly maintain cash balances at third-party financial institutions, including formerly with Silicon Valley Bank, both in the United States and internationally, in excess of the FDIC insurance limit and similar regulatory insurance limits outside the United States. Further, if we enter into a credit, loan or other similar facility with a financial institution, certain covenants included in such facility may require as security that we keep a significant portion of our cash with the institution providing such facility. If a depository institution where we maintain deposits fails or is subject to adverse conditions in the financial or credit markets, we may not be able to recover all, if any, of our deposits which could adversely impact our operating liquidity and financial performance.
On March 10, 2023, SVB was closed by the California Department of Financial Protection and Innovation, which also appointed the FDIC as receiver. Under the terms of our Convertible Debt Agreement, we are required to keep substantially all of our cash and investments with SVB. On March 12, 2023, the FDIC announced that all depositors of the bank would have access to all funds starting on March 13, 2023. As of March 13, 2023, we were afforded full access to our cash and cash equivalents with SVB. Further, on March 26, 2023, it was announced that First Citizens would assume all of SVB’s deposits and loans as of March 27, 2023. While our deposits are backed by the FDIC, that support may not last or be honored in the future and we could be materially impacted.
Risks Related to the Development of Our Product Candidate Cytisinicline
Cytisinicline is currently our sole product candidate and there is no guarantee that we will be able to successfully develop and commercialize cytisinicline.
We are currently dependent on the potential development of a single product candidate, cytisinicline. We are still developing our sole product candidate, and cytisinicline cannot be marketed or sold in the United States or in foreign markets until regulatory approval has been obtained from the FDA or applicable foreign regulatory agencies. The process of obtaining regulatory approval is expensive and time consuming. The FDA and foreign regulatory authorities may never approve cytisinicline for sale and marketing, and even if cytisinicline is ultimately approved, regulatory approval may be delayed or limited in the United States or in other jurisdictions. Even if we are authorized to sell and market cytisinicline in one or more markets, there is no assurance that we will be able to successfully market cytisinicline or that cytisinicline will achieve market acceptance sufficient to generate profits. If we are unable to successfully develop and commercialize cytisinicline due to failure to obtain regulatory approval for cytisinicline, to successfully market cytisinicline, to generate profits from the sale of cytisinicline, or due to other risk factors outlined in this report, it would have material adverse effects on our business, financial condition, and results of operations as cytisinicline is currently our sole product candidate.
Results of earlier clinical trials of cytisinicline are not necessarily predictive of future results, and any advances of cytisinicline into clinical trials may not have favorable results or receive regulatory approval.
Even if our clinical trials are completed as planned, we cannot be certain that their results will be consistent with the results of the earlier clinical trials of cytisinicline. Positive results in non-clinical testing and past clinical trials with respect to the safety and efficacy of cytisinicline do not ensure that results from subsequent clinical trials will also be positive, and we cannot be sure that the results of subsequent clinical trials will replicate the results of prior clinical trials and non-clinical testing. Any such failure may cause us to abandon cytisinicline, which would negatively affect our ability to generate any product revenues.
36
We are dependent upon a single company for the manufacture and supply of cytisinicline.
Our single product candidate, cytisinicline, has been in-licensed from a third party. We are required to continue to contract with Sopharma, to continue our development of, and potential commercialization of, cytisinicline pursuant to a supply agreement with Sopharma. Sopharma currently manufactures all of its cytisinicline API in its facilities in Bulgaria. The conflict in Ukraine, including the possibility of expanded regional or global conflict and related economic sanctions, may have negative impacts on Sopharma’s business, which could cause them to reduce or terminate investments in the cytisinicline program. If the supply agreement with Sopharma is terminated, we will need to develop or acquire alternative supply and manufacturing capabilities for cytisinicline, which we may not be able to do on commercially viable terms or at all.
Clinical trials are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Clinical development is expensive, time consuming and involves significant risk. We cannot guarantee that any clinical trial will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of development. Events that may prevent successful or timely completion of clinical development include, but are not limited to:
Any inability to successfully complete clinical development and obtain regulatory approval for cytisinicline could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to cytisinicline, we may need to conduct additional non-clinical trials, or the results obtained from such new formulation may not be consistent with previous results obtained. Clinical trial delays could also shorten any periods during which our products have patent protection and may allow competitors to develop and bring products to market before we do, which could impair our ability to successfully commercialize cytisinicline and may harm our business and results of operations.
37
Cytisinicline may cause undesirable side effects or have other properties that could delay or prevent regulatory approval, limit the commercial viability of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by cytisinicline could cause us or regulatory authorities to interrupt, delay, or terminate clinical trials or even if approved, result in a restrictive label or delay regulatory approval by the FDA or comparable foreign authorities.
Failure to reach agreement with the FDA on acceptable intake levels for impurities, such as nitrosamines, or exceeding agreed upon levels could delay or prevent regulatory approval.
Additionally, even if cytisinicline receives marketing approval, and we or others later identify undesirable side effects caused by cytisinicline, potentially significant negative consequences could result, including but not limited to:
Any of these events could prevent us from achieving or maintaining market acceptance of cytisinicline, even if approved, and could significantly harm our business, results of operations, and prospects.
The development of our product candidate is dependent upon securing sufficient quantities of cytisinicline from trees and other plants, which grows outside of the United States in a limited number of locations.
The therapeutic component of our product candidate, cytisinicline, is derived from the seeds of the Laburnum anagyroides trees and other plants, which grows in the mountains of Southern Europe and other limited locations around the world. We currently secure cytisinicline exclusively from Sopharma, a Bulgarian third-party supplier. Our current supply agreement with Sopharma expires on July 28, 2037, unless extended by mutual agreement of us and Sopharma. There can be no assurances that Laburnum anagyroides trees and other plants will continue to grow in sufficient quantities to meet commercial supply requirements or that the countries from which we can secure them will continue to allow the exportation of cytisinicline. For example, Laburnum anagyroides trees take approximately four to six years to reach maturity for harvesting and have a productive life expectancy of 20 to 25 years. There is no guarantee that Sopharma will plant sufficient trees or secure sufficient quantities of cytisinicline drug product to manage supply for our markets or to meet our forecasts. Additionally, economic or political instability or disruptions, such as the conflict in Ukraine, could negatively affect our supply chain or increase our costs. If these types of events or disruptions continue to occur, they could have a material adverse effect on our business, financial condition, results of operations and cash flows. In the event we are no longer able to obtain cytisinicline from Sopharma, or in sufficient quantities, we may not be able to produce our proposed products and our business will be adversely affected.
Our product development program may not uncover all possible adverse events that patients who take cytisinicline or our other product candidates may experience. The number of subjects exposed to cytisinicline or our other product candidates and the average exposure time in the clinical development program may be inadequate to detect rare adverse events, or chance findings, that may only be detected once the product is administered to more patients and for greater periods of time.
Clinical trials by their nature utilize a sample of the potential patient population. We cannot be fully assured that rare and severe side effects of cytisinicline will be uncovered. Such rare and severe side effects may only be uncovered with a significantly larger number of patients exposed to cytisinicline or over a significantly longer period of time. If such safety problems occur or are identified after cytisinicline reaches the market in the United States, or if such safety problems occur or are identified in foreign markets where cytisinicline is currently marketed, the FDA may require that we amend the labeling of cytisinicline or recall it, or may even withdraw approval for cytisinicline.
If the use or misuse of cytisinicline harms patients, or is perceived to harm patients even when such harm is unrelated to cytisinicline, our regulatory approvals, if any, could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims. If we are unable to obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage, a material liability claim could adversely affect our financial condition.
The use or misuse of cytisinicline in clinical trials and the sale of cytisinicline if marketing approval is obtained, exposes us to the risk of potential product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product. There is a risk that cytisinicline may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs.
38
In addition, during the course of treatment, patients may suffer adverse events for reasons that may be related to cytisinicline. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured patients, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market cytisinicline, if any, or require us to suspend or abandon our commercialization efforts. Even in a circumstance in which an adverse event is unrelated to cytisinicline, an investigation into such circumstance may be time-consuming or inconclusive. Such investigations may delay our regulatory approval process or impact and limit the type of regulatory approvals cytisinicline receives or maintains. As a result, a product liability claim, even if successfully defended, could have a material adverse effect on our business, financial condition or results of operations.
If we obtain marketing approval for cytisinicline, we will need to expand our insurance coverage to include the sale of commercial products. We cannot know if we will be able to continue to obtain product liability coverage and obtain expanded coverage if we require it, in sufficient amounts to protect us against losses due to liability, on acceptable terms, or at all. We may not have sufficient resources to pay for any liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage.
Where we have provided indemnities in favor of third parties under our agreements with them, there is a risk that these third parties could incur liability and bring a claim under such indemnities. An individual may also bring a product liability claim against us alleging that cytisinicline causes, or is claimed to have caused, an injury or is found to be unsuitable for consumer use. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts.
Any product liability claim brought against us, with or without merit, could result in:
Product liability claims may subject us to the foregoing and other risks, which could have a material adverse effect on our business, financial condition or results of operations.
Our business may be negatively affected by weather conditions, natural disasters, and the availability of natural resources, as well as by climate change.
In recent years, extreme weather events and changing weather patterns such as storms, flooding, drought, and temperature changes appear to have become more common. The production of cytisinicline from the Laburnum anagyroides and other plant depends on the availability of natural resources, including sufficient rainfall. Our exclusive supplier of cytisinicline, Sopharma, could be adversely affected if it experiences a shortage of fresh water due to droughts or if it experiences other adverse weather conditions. The long-term effects of climate change on general economic conditions and the pharmaceutical industry in particular are unclear and may heighten or intensify existing risk of natural disasters. As a result of such events, we could experience cytisinicline shortages from Sopharma, which could have a material adverse effect on our business, financial condition and results of operations.
In addition, the manufacturing and other operations of Sopharma are located near earthquake fault lines in Sofia, Bulgaria. In the event of a major earthquake, we could experience business interruptions from the disruption of our cytisinicline supplies, which could have a material adverse effect on our business, financial condition and results of operations.
39
We may conduct clinical trials internationally, which may trigger additional risks.
Conducting clinical trials in Europe or other countries outside of the United States will have additional regulatory requirements that we will have to meet in connection with our manufacturing, distribution, use of data and other matters. Failure to meet such regulatory requirements could delay our clinical trials, the approval, if any, of cytisinicline by the FDA or other regulatory authorities, or the commercialization of cytisinicline, or result in higher costs or deprive us of potential product revenues.
We may use our financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and human resources, we may forego or delay pursuit of opportunities with some programs or product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or more profitable market opportunities. Our spending on current and future research and development programs and future product candidates for specific indications may not yield any commercially viable products. We may also enter into additional strategic collaboration agreements to develop and commercialize some of our programs and potential product candidates in indications with potentially large commercial markets. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaborations, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement.
Risks Related to Regulatory Approval of Cytisinicline and Other Legal Compliance Matters
If we do not obtain the necessary regulatory approvals in the United States and/or other countries, we will not be able to sell cytisinicline.
We will need approval from the FDA to commercialize cytisinicline in the United States and approvals from similar regulatory authorities in foreign jurisdictions to commercialize cytisinicline in those jurisdictions. In order to obtain FDA approval of cytisinicline, we must submit an NDA to the FDA, demonstrating that cytisinicline is safe, pure and potent, and effective for its intended use. This demonstration requires significant research including completion of clinical trials. Satisfaction of the FDA’s regulatory requirements typically takes many years, depending upon the type, complexity and novelty of the product candidate and requires substantial resources for research, development and testing. We cannot predict whether our clinical trials will demonstrate the safety and efficacy of cytisinicline or if the results of any clinical trials will be sufficient to advance to the next phase of development or for approval from the FDA. We also cannot predict whether our research and clinical approaches will result in data that the FDA considers safe and effective for the proposed indications of cytisinicline. The FDA has substantial discretion in the product approval process. The approval process may be delayed by changes in government regulation, future legislation or administrative action or changes in FDA policy that occur prior to or during our regulatory review. Even if we comply with all FDA requests, the FDA may ultimately reject one or more of our applications. We may never obtain regulatory approval for cytisinicline. Failure to obtain approval from the FDA or comparable regulatory authorities in foreign jurisdictions to commercialize cytisinicline will leave us without saleable products and therefore without any source of revenues. In addition, the FDA may require us to conduct additional clinical testing or to perform post-marketing studies, as a condition to granting marketing approval of a product or permit continued marketing, if previously approved. If conditional marketing approval is obtained, the results generated after approval could result in loss of marketing approval, changes in product labeling, and/or new or increased concerns about the side effects or efficacy of a product. The FDA has significant post-market authority, including the explicit authority to require post-market studies and clinical trials, labeling changes based on new safety information and compliance with FDA-approved risk evaluation and mitigation strategies. The FDA’s exercise of its authority has in some cases resulted, and in the future could result, in delays or increased costs during product development, clinical trials and regulatory review, increased costs to comply with additional post-approval regulatory requirements and potential restrictions on sales of approved products. In foreign jurisdictions, the regulatory approval processes generally include the same or similar risks as those associated with the FDA approval procedures described above. We cannot be certain that we will receive the approvals necessary to commercialize cytisinicline for sale either within or outside the United States.
Even if we obtain regulatory approval for cytisinicline, we will remain subject to ongoing regulatory requirements in connection with the sale and distribution of cytisinicline.
Even if cytisinicline is approved by the FDA or comparable foreign regulatory authorities, we will be subject to ongoing regulatory requirements with respect to manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing clinical trials, and submission of safety, efficacy and other post-approval information, including both federal and
40
state requirements in the United States and the requirements of comparable foreign regulatory authorities. Compliance with such regulatory requirements will likely be costly and the failure to comply would likely result in penalties, up to and including, the loss of such approvals from the FDA or comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to continuously comply with FDA and comparable foreign regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to current cGMP regulations and corresponding foreign regulatory manufacturing requirements. As such, we, Sopharma and other contract manufacturers, if any, will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any NDA or marketing authorization application. If Sopharma or our other contract manufacturers fail to maintain cGMP compliance or fail inspections with FDA and other regulators, then our business could severely be harmed.
Ongoing post-approval monitoring and clinical trial obligations may be costly to us and the failure to meet such obligations may result in the withdrawal of such approvals.
Any regulatory approvals that we receive for cytisinicline, if any, may be subject to limitations on the approved indicated uses for which cytisinicline may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of cytisinicline. We will be required to report adverse reactions and production problems, if any, to the FDA and comparable foreign regulatory authorities. Any new legislation addressing product safety issues could result in delays in product development or commercialization, or increased costs to assure compliance. If our original marketing approval for cytisinicline was obtained through an accelerated approval pathway, we could be required to conduct a successful post-marketing clinical trial in order to confirm the clinical benefit for our products. An unsuccessful post-marketing clinical trial or failure to complete such a trial could result in the withdrawal of marketing approval.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, the regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
Any government investigation of alleged violations of law would be expected to require us to expend significant time and resources in response and could generate adverse publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to develop and commercialize our products and the value of us and our operating results would be adversely affected.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for cytisinicline and begin commercializing it in the United States, our operations may be subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations. These laws may impact, among other things, our proposed sales, marketing, and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
41
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent healthcare reform legislation has strengthened these laws. For example, the Healthcare Reform Law, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the Healthcare Reform Law provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and its results of operations.
Healthcare legislative and executive reform measures may have a material adverse effect on our business, financial condition or results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the Healthcare Reform Law was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly impacts the U.S. pharmaceutical industry. The Healthcare Reform Law, among other things, addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for products that are inhaled, infused, instilled, implanted, or injected, increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations, establishes annual fees and taxes on manufacturers of specified branded prescription products, and promotes a new Medicare Part D coverage gap discount program.
There have also been multiple recent U.S. congressional inquiries and proposed and adopted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs and biologics. In addition, Congress and multiple presidential administrations have indicated that they will continue to seek new legislative and/or administrative measures to control drug costs. These initiatives recently culminated in the enactment of the Inflation Reduction Act, or the IRA, in August 2022, which will, among other things, allow HHS to negotiate the selling price of certain drugs and biologics that CMS reimburses under Medicare Part B and Part D, although only high-expenditure single-source drugs that have been approved for at least 7 years (11 years for
42
biologics) can be selected by CMS for negotiation, with the negotiated price taking effect two years after the selection year. The negotiated prices, which will first become effective in 2026, will be capped at a statutory ceiling price beginning in October 2023, penalize drug manufacturers that increase prices of Medicare Part B and Part D drugs at a rate greater than the rate of inflation. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. Manufacturers that fail to comply with the IRA may be subject to various penalties, including civil monetary penalties. The IRA also extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. These provisions will take effect progressively starting in 2023, although they may be subject to legal challenges. We anticipate that additional state and federal healthcare measures could be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand or lower pricing for cytisinicline, or additional pricing pressures. Currently, the Healthcare Reform Law provides coverage for smoking cessation-related activities, including two counseling attempts for smoking cessation per year and medications for smoking cessation. If these provisions are repealed, in whole or in part, our business, financial condition, or results of operations could be negatively affected.
Our ability to obtain services, reimbursement or funding may be impacted by possible reductions in federal spending in the United States as well as globally.
U.S. federal government agencies currently face potentially significant spending reductions. Under the Budget Control Act of 2011, the failure of Congress to enact deficit reduction measures of at least $1.2 trillion for the years 2013 through 2021 triggered automatic cuts to most federal programs. These cuts include aggregate reductions to Medicare payments to providers of up to two percent per fiscal year, which went into effect beginning on April 1, 2013 and will stay in effect through 2025 unless additional Congressional action is taken. The American Taxpayer Relief Act of 2012, which was enacted on January 1, 2013, among other things, reduced Medicare payments to several providers, including hospitals and imaging centers. The full impact on our business of these automatic cuts is uncertain.
If government spending is reduced, anticipated budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA or the National Institutes of Health to continue to function at current levels. Amounts allocated to federal grants and contracts may be reduced or eliminated. These reductions may also impact the ability of relevant agencies to timely review and approve drug research and development, manufacturing, and marketing activities, which may delay our ability to develop, market and sell any products we may develop. Any reductions in government spending in countries outside the United States may also impact us negatively, such as by limiting the functioning of international regulatory agencies in countries outside the United States or by eliminating programs on which we may rely.
In July 2021, we announced that we were awarded a grant from NIDA to evaluate the use of cytisinicline as a treatment for cessation of nicotine e-cigarette use. This initial grant award, in the amount of $320,000, commenced on August 1, 2021, and was utilized to complete critical regulatory and clinical operational activities, such as protocol finalization, clinical trial site identification, and submission of an IND application to the FDA for investigating cytisinicline in nicotine e-cigarette users. In November 2021, we announced that the FDA had completed their review and accepted the IND application to investigate cytisinicline as a cessation treatment in this population. In June 2022, following NIDA/NIH review of completed milestones, we announced that we were awarded the next grant funding from NIDA in the amount of approximately $2.5 million to conduct the ORCA-V1 Phase 2 clinical study evaluating cytisinicline in 160 adult nicotine e-cigarette users in the United States. The full grant award of $2.8 million is expected to cover approximately half of the ORCA-V1 clinical study costs. If amounts allocated to federal grants were reduced or eliminated, we would be required to fund the shortfall in the ORCA-V1 clinical study costs, which may result in delay of initiation of or cancellation of the study.
Our employees, independent contractors, consultants, commercial partners, principal investigators, or CROs may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of fraud or misconduct by employees, independent contractors, consultants, commercial partners, principal investigators, or CROs, which could include intentional, reckless, negligent, or unintentional failures to comply with FDA regulations, comply with applicable fraud and abuse laws, provide accurate information to the FDA, report financial information or data accurately, or disclose unauthorized activities to us. This misconduct could also involve the improper use or misrepresentation of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter this type of misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, financial condition and results of operations, including the imposition of significant fines or other sanctions. Further, even if we are successful in asserting a defense, we may incur substantial costs in preparing and maintaining our defense and any such
43
action would be time- and resource-intensive and potentially divert management’s attention from the business, which could adversely affect our business and results of operations.
Risks Related to our Business Operations
It is difficult to evaluate our current business, predict our future prospects and forecast our financial performance and growth.
To date our business activities have been focused primarily on the development and regulatory approval of cytisinicline and its various alternative forms. Although we have not generated revenue to date, we expect that, after any regulatory approval, any receipt of revenue will be attributable to sales of cytisinicline, primarily in the United States, the EU (including the U.K.) and Asia. Because we devote substantially all of our resources to the development of cytisinicline and rely on cytisinicline as our sole source of potential revenue for the foreseeable future, any factors that negatively impact this product, or result in decreasing product sales, would materially and adversely affect our business, financial condition and results of operations.
Our future success depends in part on our ability to attract, retain, and motivate other qualified personnel.
We will need to expand and effectively manage our managerial, operational, financial, development and other resources in order to successfully pursue our development and commercialization efforts for our existing and future product candidates. We expect to need additional scientific, technical, operational, financial and other personnel. Our success depends on our continued ability to attract, retain and motivate highly qualified personnel, such as management, clinical and preclinical personnel, including our executive chairman Richard Stewart and our executive officers John Bencich, Cindy Jacobs, Anthony Clarke and Jaime Xinos. In addition, although we have entered into employment agreements with each of Mr. Stewart, Mr. Bencich, Dr. Jacobs, Dr. Clarke and Ms. Xinos, such agreements permit those executives to terminate their employment with us at any time, subject to providing us with advance written notice.
We may not be able to attract and retain personnel on acceptable terms, if at all, given the competition among numerous pharmaceutical and biotechnology companies for individuals with similar skill sets. In addition, failure to succeed in development and commercialization of cytisinicline may make it more challenging to recruit and retain qualified personnel. The inability to recruit and retain qualified personnel, or the loss of the services of our current personnel may impede the progress of our research, development, and commercialization objectives and would negatively impact our ability to succeed in our product development strategy.
We will need to expand our organization and we may experience difficulties in managing this growth, which could disrupt our operations.
We may need to expand our organization, which may require us to divert a disproportionate amount of our attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in its infrastructure, operational mistakes, loss of business opportunities, loss of employees, and reduced productivity among remaining employees. Expanded growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If we are unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth.
In the future, we may invest in the development of additional indications for cytisinicline. If we invest in and are unsuccessful in developing additional indications for cytisinicline, our business, financial condition and results of operations may be adversely affected.
In the future, we may invest in the research and development of new indications for cytisinicline to address nicotine addictions associated with the use of e-cigarette, or vaping, products. Given their recent introduction, the use of vaping products is not fully understood which may increase the risk of failure in this area. We are pursuing clinical studies in users of e-cigarettes and have been awarded a grant by the NIDA/NIH to evaluate the use of cytisinicline as a treatment for cessation of nicotine e-cigarette use. Continued grant funding under the award will still be subject to availability of funds at the NIDA/NIH, and such funding will not be sufficient to cover the full clinical costs of the Phase 2 ORCA-V1 trial. We expect that we will need to invest significant amounts of capital to pursue development of an e-cigarette cessation indication. If we are unable to provide such additional capital when needed, we may be unable to complete the development, regulatory approval and commercialization of an e-cigarette cessation indication.
The development of additional indications for cytisinicline is highly uncertain. During the research and development cycle, we may expend significant time and resources on developing additional indications without any assurance that we will recoup our investments
44
or that our efforts will be commercially successful. A high rate of failure is inherent in the discovery and development of additional indications, and failure can occur at any point in the process, including late in the process after substantial investment. Further, any new indications may not be accepted by physicians and the medical community at large, and competitors may develop and market equivalent or superior products. Failure to launch commercially successful new indications for cytisinicline after significant investment could have a material adverse effect on our business, financial condition and results of operations.
Our internal computer systems, or those of our third-party collaborators or other service providers, may fail or suffer security breaches and cyber-attacks, which could result in a material disruption of our development programs.
We believe that we take reasonable steps that are designed to protect the security, integrity and confidentiality of the information we collect, use, store, and disclose, but inadvertent or unauthorized data access may occur despite our efforts. For example, our system protections may be ineffective or inadequate, or we could be impacted by software bugs or other technical malfunctions, as well as employee error or malfeasance. Additionally, privacy and data protection laws are evolving, and it is possible that these laws may be interpreted and applied in a manner that is inconsistent with our data handling safeguards and practices that could result in fines, lawsuits, and other penalties, and significant changes to our or our third-party collaborators or service providers business practices and products and service offerings. To the extent that the measures we or our third-party collaborators or service providers have taken prove to be insufficient or inadequate, we may become subject to litigation, breach notification obligations, or regulatory or administrative sanctions, which could result in significant fines, penalties, damages, harm to our reputation, or loss of customers. While we have not experienced any material losses as a result of any system failure, accident or security breach to date, we have been the subject of certain phishing attempts in the past. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information or other similar disruptions. Additionally, a party who circumvents our security measures could, among other effects, appropriate patient information or other proprietary data, cause interruptions in our operations, or expose our collaborators to hacks, viruses, and other disruptions. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. In addition, insurance coverage to compensate for any losses associated with such events may not be adequate to cover all potential losses. The development and maintenance of these systems, controls and processes is costly and requires ongoing monitoring and updating as technologies change and efforts to overcome security measures become increasingly sophisticated.
To the extent that any disruption, security breach, or cyber-attack were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of personal, confidential or proprietary information, we could incur liability, our competitive position could be harmed and the further development and commercialization of our product candidates could be delayed. Depending on the nature of the information compromised, in the event of a data breach or other unauthorized access to our patient data, we may also have obligations to notify patients and regulators about the incident, and we may need to provide some form of remedy, such as a subscription to credit monitoring services, pay significant fines to one or more regulators, or pay compensation in connection with a class-action settlement (including under the new private right of action under the California Consumer Privacy Act of 2018, which is expected to increase security breach litigation). Such breach notification laws continue to evolve and may be inconsistent from one jurisdiction to another. Complying with these obligations could cause us to incur substantial costs and could increase negative publicity surrounding any incident that compromises customer data. Additionally, the financial exposure from the events referenced above could either not be insured against or not be fully covered through any insurance that we may maintain, and there can be no assurance that the limitations of liability in any of our contracts would be enforceable or adequate or would otherwise protect us from liabilities or damages as a result of the events referenced above. Any of the foregoing could have an adverse effect on our business, reputation, financial condition and results of operations.
Risks Related to Our Reliance on Third Parties
We expect to continue to rely on third parties to manufacture cytisinicline for use in clinical trials, and we intend to exclusively rely on Sopharma to produce and process cytisinicline, if approved. Our commercialization of cytisinicline could be stopped, delayed or made less profitable if Sopharma fails to obtain approval of government regulators, fails to provide us with sufficient quantities of product, or fails to do so at acceptable quality levels or prices.
We do not currently have, nor do we currently plan to develop, the infrastructure or capability internally to manufacture our clinical supplies for use in the conduct of our clinical trials, and we lack the resources and the capability to manufacture cytisinicline on a clinical or commercial scale. We currently exclusively rely on Sopharma to manufacture cytisinicline for use in clinical trials and plan to continue relying on Sopharma to manufacture cytisinicline on a commercial scale, if approved.
45
Our reliance on Sopharma exposes us to the following additional risks:
Each of these risks could delay our clinical trials, the approval, if any of cytisinicline by the FDA or the commercialization of cytisinicline or result in higher costs or deprive us of potential product revenue.
We rely on third party contract manufacturing organizations, or CMOs, to package the cytisinicline used in our clinical trials. If any of these CMO’s fail to timely deliver the supplies needed, then our clinical studies could be delayed materially. Third-party manufacturers may fail to perform under their contractual obligations, or may fail to deliver the required commercial product on a timely basis and at commercially reasonable prices. If we are required to identify and qualify an alternate manufacturer, we may be forced to delay or suspend our clinical trials. We expect to continue to depend on third-party contract manufacturers for the foreseeable future.
The manufacture of medical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of medical products often encounter difficulties in production, particularly in scaling up and validating initial production and absence of contamination. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if contaminants are discovered in the supply of cytisinicline or in the Sopharma manufacturing facilities, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. We cannot be assured that any stability or other issues relating to the manufacture of cytisinicline will not occur in the future. Additionally, Sopharma may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or political instability in the countries in which Sopharma conducts its operations. For example, the military conflict between Russia and Ukraine may increase the likelihood of supply interruptions and hinder our ability to find the materials we need to make our product candidate. If Sopharma were to encounter any of these difficulties, or otherwise fail to comply with its contractual obligations, our ability to provide our product candidate to patients in clinical trials could be delayed or suspended. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely. Similar political instability could also harm the commercial production and supply of cytisinicline in the event that cytisinicline is ultimately approved for commercial sale.
In June 2021, Pfizer Inc. halted the distribution of its smoking cessation drug, Chantix (varenicline), after heightened levels, above the FDA’s acceptable daily intake limit, of nitrosamines were found in some lots of Chantix pills. In September 2021, Pfizer announced a nationwide recall in the United States of all lots of Chantix and have also withdrawn the product in other countries around the globe. We have undertaken a review of cytisinicline in accordance with regulatory guidance to assess the risk of the presence of nitrosamine and other potential impurities. If contaminants, or impurities such as nitrosamines, are discovered in quantities above regulators’ thresholds within our supply of cytisinicline, we may potentially delay product development and approval or have a material adverse impact on our business.
46
We rely on third parties to conduct our clinical trials and perform other services. If these third parties do not successfully perform and comply with regulatory requirements, we may not be able to successfully complete clinical development, obtain regulatory approval or commercialize cytisinicline and our business could be substantially harmed.
We plan to rely upon third-party CROs to conduct, monitor and manage our ongoing clinical programs. We rely on these parties for execution of clinical trials and manage and control only some aspects of their activities. We remain responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs and other vendors are required to comply with all applicable laws, regulations and guidelines, including those required by the FDA and comparable foreign regulatory authorities for all of our product candidates in clinical development. If we or any of our CROs or vendors fail to comply with applicable laws, regulations and guidelines, the results generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot be assured that our CROs and other vendors will meet these requirements, or that upon inspection by any regulatory authority, such regulatory authority will determine that efforts, including any of our clinical trials, comply with applicable requirements. Our failure to comply with these laws, regulations and guidelines may require us to repeat clinical trials, which would be costly and delay the regulatory approval process.
If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs in a timely manner or do so on commercially reasonable terms. In addition, our CROs may not prioritize our clinical trials relative to those of other customers and any turnover in personnel or delays in the allocation of CRO employees by the CRO may negatively affect our clinical trials. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, continued development of cytisinicline may be delayed or terminated and we may not be able to meet our current plans with respect to cytisinicline. CROs may also involve higher costs than anticipated, which could negatively affect our financial condition and operations.
We may not be able to establish or maintain the third-party relationships that are necessary to develop or potentially commercialize cytisinicline.
Our business plan relies heavily on third party collaborators, partners, licensees, clinical research organizations, clinical investigators, vendors or other third parties to support our research and development efforts and to conduct clinical trials for cytisinicline. We cannot guarantee that we will be able to successfully negotiate agreements for, or maintain relationships with, these third parties on a commercially reasonable basis, if at all. If we fail to establish or maintain such third-party relationships as anticipated, our business could be adversely affected.
We may be unable to realize the potential benefits of any collaborations which we may enter into with other companies for the development and commercialization of cytisinicline.
We may enter into a collaboration with third parties concerning the development and/or commercialization of cytisinicline; however, there is no guarantee that any such collaboration will be successful. Collaborations may pose a number of risks, including:
47
As a result, a collaboration may not result in the successful development or commercialization of cytisinicline.
We enter into various contracts in the normal course of our business in which we indemnify the other party to the contract. In the event we have to perform under these indemnification provisions, it could have a material adverse effect on our business, financial condition and results of operations.
In the normal course of business, we enter into academic, commercial, service, collaboration, licensing, consulting and other agreements that contain indemnification provisions. With respect to our academic and other research agreements, we typically indemnify the institution and related parties from losses arising from claims relating to the products, processes or services made, used, sold or performed pursuant to the agreements for which we have secured licenses, and from claims arising from our or our sublicensees’ exercise of rights under the agreement. With respect to our collaboration agreements, we indemnify our collaborators from any third-party product liability claims that could result from the production, use or consumption of the product, as well as for alleged infringements of any patent or other intellectual property right by a third party. With respect to consultants, we indemnify them from claims arising from the good faith performance of their services.
Should our obligation under an indemnification provision exceed applicable insurance coverage or if we were denied insurance coverage, our business, financial condition and results of operations could be adversely affected. Similarly, if we are relying on a collaborator to indemnify us and the collaborator is denied insurance coverage or the indemnification obligation exceeds the applicable insurance coverage, and if the collaborator does not have other assets available to indemnify us, our business, financial condition and results of operations could be adversely affected.
We may rely on third parties to perform many essential services for any of our current or future product candidates that we commercialize, including services related to warehousing and inventory control, distribution, government price reporting, customer service, accounts receivable management, cash collection, and adverse event reporting. If these third parties fail to perform as expected or to comply with legal and regulatory requirements, our ability to commercialize any of our current or future product candidates will be significantly impacted and we may be subject to regulatory sanctions.
We may retain third‑party service providers to perform a variety of functions related to the sale and distribution of any of our current or future product candidates, key aspects of which will be out of our direct control. These service providers may provide key services related to warehousing and inventory control, distribution, government price reporting, customer service, accounts receivable management, and cash collection, and, as a result, most of our inventory may be stored at a single warehouse maintained by one such service provider. If we retain a service provider, we would substantially rely on it as well as other third‑party providers that perform services for us, including entrusting our inventories of products to their care and handling. If these third‑party service providers fail to comply with applicable laws and regulations, fail to meet expected deadlines, or otherwise do not carry out their contractual duties to us, or encounter physical or natural damage at their facilities, our ability to deliver product to meet commercial demand would be significantly impaired and we may be subject to regulatory enforcement action.
In addition, we may engage third parties to perform various other services for us relating to adverse event reporting, safety database management, fulfillment of requests for medical information regarding our product candidates and related services. If the quality or accuracy of the data maintained by these service providers is insufficient, or these third parties otherwise fail to comply with regulatory requirements related to adverse event reporting, we could be subject to regulatory sanctions.
Additionally, if a third-party errs in calculating government pricing information from transactional data in our financial records, it could impact our discount and rebate liability and potentially cause government programs to overpay providers for our products, which could expose us to significant False Claims Act liability and other civil monetary penalties.
48
Risks Related to Commercialization of Cytisinicline
We face substantial competition and our competitors may discover, develop or commercialize products faster or more successfully than us.
The development and commercialization of new products is highly competitive. We face competition from major pharmaceutical companies, specialty pharmaceutical companies, biotechnology companies, universities and other research institutions worldwide with respect to products for smoking cessation and other product candidates that we may seek to develop or commercialize in the future. We are aware that many companies have therapeutics marketed or in development for smoking cessation. We expect that our competitors and potential competitors have historically dedicated, and will continue to dedicate, significant resources to aggressively develop and commercialize their products in order to take advantage of the significant market opportunity.
Many of our competitors have substantially greater financial, name recognition, manufacturing, marketing, research, technical and other resources than us. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Further, our competitors may develop new products that are safer, more effective or more cost-efficient than cytisinicline. Large pharmaceutical companies in particular have extensive expertise in non-clinical and clinical testing and in obtaining regulatory approvals for products. In addition, academic institutions, government agencies, and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies. These organizations may also establish exclusive collaborative or licensing relationships with our competitors. Failure of cytisinicline to effectively compete against established treatment options or in the future with new products currently in development would harm our business, financial condition, results of operations and prospects.
The commercial success of cytisinicline will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community. Failure to obtain or maintain adequate reimbursement or insurance coverage for products, if any, could limit our ability to market cytisinicline and decrease our ability to generate revenue.
Even with the approvals from the FDA and comparable foreign regulatory authorities, the commercial success of cytisinicline will depend in part on the healthcare providers, patients, and third-party payors accepting cytisinicline as medically useful, cost-effective, and safe. Cytisinicline may not gain market acceptance by physicians, patients and third-party payors. The degree of market acceptance of cytisinicline will depend on a number of factors, including but not limited to:
Even if cytisinicline displays a favorable efficacy and safety profile upon approval, market acceptance of cytisinicline remains uncertain. Efforts to educate the medical community and third-party payors on the benefits of cytisinicline, if any, may require significant investment and resources and may never be successful. Additionally, third-party payors, including governmental and private insurers, may also encourage the use of generic products instead of cytisinicline, or a generic version of cytisinicline, which require a prescription or may be available OTC. If our products fail to achieve an adequate level of acceptance by physicians, patients, third-party payors, and other healthcare providers, we will not be able to generate sufficient revenue to become or remain profitable.
The pricing, coverage, and reimbursement of cytisinicline, if any, must be sufficient to support our commercial efforts and other development programs and the availability and adequacy of coverage and reimbursement by third-party payors, including governmental and private insurers, are essential for most patients to be able to afford treatments. Sales of cytisinicline, if any, will depend substantially, both domestically and abroad, on the extent to which the costs of cytisinicline will be paid for or reimbursed by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or government payors and
49
private payors. If coverage and reimbursement are not available, or are available only in limited amounts, we may have to subsidize or provide cytisinicline for free or we may not be able to successfully commercialize cytisinicline.
In addition, there is significant uncertainty related to the insurance coverage and reimbursement for newly approved products. In the United States, the principal decisions about coverage and reimbursement for new products are typically made by the Centers for Medicare and Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new product will be covered and reimbursed under Medicare. Private payors tend to follow the coverage reimbursement policies established by CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for novel product candidates such as cytisinicline and what reimbursement codes cytisinicline may receive if approved.
Outside the United States, selling operations are generally subject to extensive governmental price controls and other price-restrictive regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada, and other countries has and will continue to put pressure on the pricing and usage of products. In many countries, the prices of products are subject to varying price control mechanisms as part of national health systems. Price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our products, if any. Accordingly, in markets outside the United States, the potential revenue may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and private payors in the United States and abroad to limit or reduce healthcare costs may result in restrictions on coverage and the level of reimbursement for new products and, as a result, they may not cover or provide adequate payment for our products. We expect to experience pricing pressures in connection with products due to the increasing trend toward managed healthcare, including the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription products, has and is expected to continue to increase in the future. As a result, profitability of cytisinicline, if any, may be more difficult to achieve even if regulatory approval is received.
Sopharma may breach its supply agreement with us and sell cytisinicline into our territories or permit third parties to export cytisinicline into our territories and negatively affect our commercialization efforts of our products in our territories.
We are currently dependent on the exclusivity provisions of our supply agreement with Sopharma to conduct our business and to prevent Sopharma from competing, directly and indirectly, with us in the United States and Western Europe. If Sopharma were to breach the exclusivity provisions of the supply agreement with us and sell or distribute cytisinicline directly into our territories or permit third parties to export cytisinicline into our territories, among other things, the increase in competition within our anticipated markets could have a material adverse effect on our business, results of operations and financial condition.
The illegal distribution and sale by third parties of counterfeit versions of cytisinicline, stolen products, or alternative third-party distribution and sale of cytisinicline could have a negative impact on our financial performance or reputation.
Cytisinicline is not eligible for composition of matter patents in the United States as it is a naturally occurring substance. As such, third parties are able to manufacture, sell or distribute cytisinicline without royalties or other payments to us and compete with our products in the United States and potentially worldwide and negatively impact our commercialization efforts of our products. We are aware of additional cytisinicline products approved in several European countries and we may not be able to block other third parties from launching generic versions of cytisinicline. Third parties may also sell or distribute cytisinicline as an herbal or homeopathic product. Other than regulatory exclusivity or other limitations, there may be little to nothing to stop these third parties from manufacturing, selling or distributing cytisinicline. Because we have no ability to set rigorous safety standards or control processes over third party manufacturers, sellers or distributors of cytisinicline, excluding Sopharma, these formulations of cytisinicline may be unsafe or cause adverse effects to patients and negatively impact the reputation of cytisinicline as a safe and effective smoking cessation aid.
Third parties could illegally distribute and sell counterfeit versions of cytisinicline, especially on online marketplaces, which do not meet the rigorous manufacturing and testing standards under cGMP. Counterfeit products are frequently unsafe or ineffective, and may even be life-threatening. Counterfeit medicines may contain harmful substances, the wrong dose of the active pharmaceutical ingredient or no active pharmaceutical ingredients at all. However, to distributors and users, counterfeit products may be visually indistinguishable from the authentic version.
Reports of adverse reactions to counterfeit products, increased levels of counterfeiting, or unsafe cytisinicline products could materially affect patient confidence in our cytisinicline product. It is possible that adverse events caused by unsafe counterfeit or other cytisinicline products that we do not produce will mistakenly be attributed to our cytisinicline product. In addition, thefts of inventory that are not properly stored at warehouses, plants or while in-transit, and which are sold through unauthorized channels could adversely impact patient safety, our reputation, and our business. Public loss of confidence in the integrity in cytisinicline as a result of counterfeiting, theft, or improper manufacturing processes could have a material adverse effect on our business, results of operations, and financial condition.
50
It is illegal to sell unapproved prescription medicines in the United States. Sopharma’s cytisinicline brand is currently approved for sale in certain Central and Eastern European countries. Cytisinicline has not yet received a marketing approval from the FDA, and we intend to conduct the requisite clinical trials to obtain approval for the marketing of cytisinicline in the United States and in major global markets. We are aware that products purporting to be Sopharma’s cytisinicline brand are available, via third party internet sites, for importation in the United States and other global markets. We have no control over the authenticity of products purchased through these sites, which may be counterfeit or sourced from distributors in Central and Eastern Europe without authorization to sell into the United States or EU.
We may attempt to form collaborations in the future with respect to cytisinicline, but we may not be able to do so, which may cause us to alter our development and commercialization plans.
We may attempt to form strategic collaborations, create joint ventures or enter into licensing arrangements with third parties with respect to our programs that we believe will complement or augment our existing business. We may face significant competition in seeking appropriate strategic collaborators, and the negotiation process to secure appropriate terms is time consuming and complex. We may not be successful in our efforts to establish such a strategic collaboration for cytisinicline on terms that are acceptable to us, or at all. This may be because cytisinicline may be deemed to be at too early of a stage of development for collaborative effort, our research and development pipeline may be viewed as insufficient, the competitive or intellectual property landscape may be viewed as too intense or risky, or cytisinicline’s patent protection insufficient, and/or third parties may not view cytisinicline as having sufficient potential for commercialization, including the likelihood of an adequate safety and efficacy profile.
Any delays in identifying suitable collaborators and entering into agreements to develop and/or commercialize cytisinicline could delay the development or commercialization of cytisinicline, which may reduce our competitiveness even if we reach the market. Absent a strategic collaborator, we would need to undertake development and/or commercialization activities at our own expense. If we elect to fund and undertake development and/or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we are unable to do so, we may not be able to develop our product candidate cytisinicline or bring it to market and our business may be materially and adversely affected.
We may not be successful in any efforts to identify, license, discover, develop, or commercialize additional product candidates.
Although a substantial amount of our effort will focus on clinical testing, approval, and potential commercialization of cytisinicline, our sole product candidate, the success of our business is also expected to depend in part upon our ability to identify, license, discover, develop, or commercialize additional product candidates. Research programs to identify new product candidates require substantial technical, financial, and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. Our research programs or licensing efforts may fail to yield additional product candidates for clinical development and commercialization for a number of reasons, including but not limited to the following:
If any of these events occur, we may be forced to abandon our development efforts for a program or programs, or we may not be able to identify, license, discover, develop, or commercialize additional product candidates, which would have a material adverse effect on our business, financial condition or results of operations and could potentially cause us to cease operations.
51
Risks Related to our Intellectual Property
We may not be successful in obtaining or maintaining necessary rights to cytisinicline, product compounds and processes for our development pipeline through acquisitions and in-licenses.
Presently, we have rights to intellectual property through trade secrets, licenses, patents from third parties, and patents and applications that we own. Our product candidate may require specific formulations to work effectively and efficiently, and these rights may be held by others. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
If we are unable to maintain effective proprietary rights for our product candidate or any future product candidates, we may not be able to compete effectively in our proposed markets.
We currently rely primarily on trade secret protection and on confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. Trade secrets can be difficult to protect, however, and even where they are protected, they generally provide less intellectual property protection to the holder of the trade secret than to a holder of a patent. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors.
Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors, and any third parties who have access to our proprietary know-how, information, or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business, financial condition or results of operations. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
We are currently developing cytisinicline for smoking cessation. Our commercial success depends in part on our ability to develop, manufacture, market and sell our product candidates and use our proprietary technology without infringing the patent rights of third parties. We are not aware of any patents or patent applications that would prevent the development, manufacture or marketing of cytisinicline for smoking cessation.
We are aware of U.S. and foreign patents and pending patent applications owned by third parties that cover certain other therapeutic uses of cytisinicline. We are currently monitoring these patents and patent applications. We may in the future pursue available proceedings in the United States and foreign patent offices to challenge the validity of these patents and patent applications. In addition, or alternatively, we may consider whether to seek to negotiate a license of rights to technology covered by one or more of such patents and patent applications for these certain additional therapeutic uses. If any third-party patents or patent applications cover our product candidates or technologies in other therapeutic uses, we may not be free to manufacture or market our product candidates for additional therapeutic uses, absent such a license, which may not be available to us on commercially reasonable terms, or at all.
It is also possible that we have failed to identify relevant third-party patents or applications. For example, applications filed before November 29, 2000 and applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Moreover, it is difficult for industry participants, including us, to identify all third-party patent rights that may be relevant to our product candidates and technologies because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. We may fail to identify relevant patents or patent applications or may identify pending patent applications of potential interest but incorrectly predict the likelihood that such
52
patent applications may issue with claims of relevance to our technology. In addition, we may be unaware of one or more issued patents that would be infringed by the manufacture, sale or use of a current or future product candidate, or we may incorrectly conclude that a third-party patent is invalid, unenforceable or not infringed by our activities. Additionally, pending patent applications that have been published can, subject to specified limitations, be later amended in a manner that could cover our technologies, our product candidates or the use of our product candidates.
There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, and reexamination proceedings before the U.S. Patent and Trademark Office, or USPTO, and corresponding foreign patent offices. U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidate. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidate may be subject to claims of infringement of the patent rights of third parties.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
We intend to rely on patent rights for certain aspects of our product candidates and certain future product candidates. If we are unable to obtain or maintain an adequate proprietary position from this approach, we may not be able to compete effectively in our markets.
Although we rely or will rely primarily on trade secret protection as part of our intellectual property rights strategies, we also intend to rely on patent rights to protect certain aspects of our technologies and upon the patent rights of third parties from which we license certain of our technologies.
We have sought to protect our proprietary position by filing patent applications in the U.K., United States and certain other countries around the world related to future product candidates. This process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner or at all. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
The patent position of pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unsolved. The patent applications that we own may fail to result in issued patents with claims that cover our product candidates in the United States or in other foreign countries. There is no assurance that all potentially relevant prior art relating to our patent applications or our patents (once issued) have been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our future product candidates, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our future product candidates, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us after patent issuance could deprive us of rights necessary for the successful commercialization of any future product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a future product candidate under patent protection could be reduced.
If we cannot obtain and maintain effective protection of exclusivity from our regulatory efforts and intellectual property rights, including patent protection or data exclusivity, for our product candidates, we may not be able to compete effectively and our business and results of operations would be harmed.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity, and is therefore costly, time-consuming, and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard
53
to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained, if any. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
In Assoc. for Molecular Pathology v. Myriad Genetics, Inc., the U.S. Supreme Court held that certain claims to naturally occurring substances are not patentable. Cytisinicline is a naturally occurring product and is not patentable. Our intellectual property strategy involves novel formulations of cytisinicline and there is no guarantee that such patents will be issued or if issued, will be broad enough to prevent competitors from developing competing cytisinicline products. Although we do not believe that any patents that may issue from our pending patent applications directed at our product candidate, if issued in their currently pending forms, as well as patent rights licensed by us, will be found invalid based on this decision, we cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patent rights. There could be similar changes in the laws of foreign jurisdictions that may impact the value of our patent rights or our other intellectual property rights.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We employ individuals who were previously employed at other biotechnology or pharmaceutical companies. Although we have written agreements and make every effort to ensure that our employees, consultants, and independent contractors do not use the proprietary information or intellectual property rights of others in their work for us, we may in the future be subject to any claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
It is difficult and costly to protect our proprietary rights and as a result we may not be able to ensure their protection. In addition, patents have a limited lifespan and will eventually expire.
Market exclusivity awarded by the FDA upon the approval of an NDA is limited in scope and duration. Our commercial success will depend in part on obtaining, maintaining, enforcing, and defending against third‑party challenges, patent and trade secret protection for our current and future product candidates that we may develop, license or acquire, as well as the related manufacturing methods. We will be able to protect our technologies from unauthorized use by third parties to the extent that the technologies are covered by valid and enforceable patents or trade secrets.
The patent prosecution process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, should we enter into additional collaborations we may be required to consult with or cede control to collaborators regarding the prosecution, maintenance, and enforcement of our patent applications and patents. Therefore, these patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in pharmaceutical or biotechnology patents has emerged to date in the United States. The patent situation outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents and patent applications or in third‑party patents and patent applications. The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. Moreover, the patent application process is also subject to numerous risks and uncertainties, and there can be no assurance that we or any of our future development partners will be successful in protecting any of our current or future product candidates that we may develop, license, or acquire by obtaining and defending patents. For example:
54
Patents have a limited lifespan. In most countries, including the United States, the expiration of a patent is typically 20 years from the date that the application for the patent is filed. Various extensions of patent term may be available in particular countries; however, in all circumstances the life of a patent, and the protection it affords, has a limited term. If we encounter delays in obtaining regulatory approvals, the period of time during which we could market a product under patent protection could be reduced. We expect to seek extensions of patent terms where these are available in any countries where we are prosecuting patents. Such possible extensions include those permitted under the Drug Price Competition and Patent Term Restoration Act of 1984 in the United States, which permits a patent term extension of up to five years to cover an FDA‑approved product. The actual length of the extension will depend on the amount of patent term lost while the product was in clinical trials. However, the applicable authorities, including the USPTO and the FDA in the United States, and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data, and then may be able to launch their product earlier than might otherwise be the case.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment, and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment, and other similar provisions during the patent prosecution process. Periodic maintenance fees, renewal fees, annuity fees, and various other governmental fees on patents or patent applications will be due to be paid to the USPTO and various patent agencies outside of the United States in several stages over the lifetime of the patents and applications. We have systems in place to remind us to pay these fees, and we employ and rely on reputable law firms and other professionals to effect payment of these fees to the USPTO and non‑U.S. patent agencies for the patents and patent applications we own and those that we in‑license. We also employ reputable law firms and other professionals to help us comply with the various documentary and other procedural requirements with respect to the patents and patent applications that we own and those that we in‑license. In some cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe our issued patents, our in-licensed patents, or other intellectual property that we own or in-license. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part; construe the patent’s claims narrowly; or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
55
Most of our competitors are larger than we are and have substantially greater resources than we do. They are, therefore, likely to be able to sustain the costs of complex patent litigation longer than we could. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our internal research programs, in-license needed technology, or enter into development partnerships that would help us bring our product candidates to market.
We or our licensors may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patent applications and patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where enforcement rights are not as strong as those in the United States. These products may compete with our product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our or our licensors’ intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Common Stock
The price for our common stock is volatile.
The market prices for our common stock and that of early-stage pharmaceutical, biotechnology and other life sciences companies have historically been particularly volatile. Some of the factors that may cause the market price of our common stock to fluctuate include:
56
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of our common stock. An increase in the market price of our common stock, which is uncertain and unpredictable, may be the sole source of gain from an investment in our common stock. An investment in our common stock may not be appropriate for investors who require dividend income. We have never declared or paid cash dividends on our capital stock and do not anticipate paying any cash dividends on our capital stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be the sole source of gain for stockholders for the foreseeable future. Accordingly, an investment in our common stock may not be appropriate for investors who require dividend income or investors who are not prepared to bear a significant risk of losses from such an investment.
The issuance or sale of additional shares of our common stock may cause the price of our common stock to decline and result in dilution to our existing stockholders.
In December 2021, we entered into an At-the-Market Offering Sales Agreement, or ATM, with Virtu Americas, LLC, as sales agent, pursuant to which we may sell shares of common stock with an aggregate offering price of up to $25 million. During the three months ended March 31, 2023, we sold no shares of our common stock pursuant to the ATM. As of March 31, 2023, shares of our common stock having an aggregate value of $23.5 million remained available for sale under the ATM. Also in December 2021, we entered into a $25.0 million contingent convertible debt agreement, or Debt Agreement, with Silicon Valley Bank, or SVB, and SVB Innovation Credit Fund VIII, L.P., or, together with SVB, the Lenders, which was amended in April 2022. As part of the contingent convertible debt agreement, the Lenders funded $15.0 million in the form of convertible indebtedness, or Convertible Debt, at closing. Subject to
57
certain terms and conditions, the Lenders may convert all or any part of the outstanding Convertible Debt and accrued and unpaid interest at any time prior to maturity into shares of our common stock at a conversion price equal to $9.34 per share, subject to customary anti-dilution adjustments. Additionally, all outstanding Convertible Debt, including accrued and unpaid interest, will mandatorily convert into shares of our common stock, at the conversion price, on such date, if any, when the closing price per share of our common stock has been equal to or greater than $24.00 for 30 consecutive trading days prior to such date. On March 10, 2023, SVB was closed by the California Department of Financial Protection and Innovation, which also appointed the FDIC as receiver. We are aware that there can be no assurance that the Term Loans will be available to us for borrowing nor whether SVB or any successor lender(s) will be willing to work with the Company on any modifications to the current Convertible Debt or Term Loan agreements. Any amounts due under the Convertible Debt, including interest payments, will remain payable to any successor lender(s). In addition, we have warrants outstanding to purchase up to approximately 5,333,088 shares of common stock, at a weighted-average exercise price of $7.26 per share.
The sale of additional shares of our common stock pursuant to the ATM, the conversion of the Convertible Debt into shares of our common stock, or the exercise of any of our outstanding warrants would have a dilutive impact on our existing stockholders. Sales under the ATM, the conversion of the Convertible Debt or the exercise of any of our outstanding warrants, could cause the market price of our common stock to decline significantly. Sales of our common stock under the ATM, the conversion of the Convertible Debt, the exercise of any of our outstanding warrants or the perception that such events will occur, could also encourage short sales by third parties, which could contribute to the further decline of the price of our common stock. Additionally, the sale of a substantial number of shares of our common stock under the ATM, the conversion of the Convertible Debt, the exercise of any of our outstanding warrants or the perception that such events will occur, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish.
In addition, in the future, we plan to raise additional capital through private placements or public offerings of our equity or debt securities. We cannot be certain that additional funding will be available on acceptable terms, if at all. To the extent that we raise additional financing by issuing equity securities, we may do so at a price per share that represents a discount to the then-current per share trading price of our common stock and our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants, such as limitations on our ability to incur additional indebtedness, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely affect our ability to conduct our business.
Because our merger resulted in an ownership change under Section 382 of the U.S. Internal Revenue Code for OncoGenex, pre-merger net operating loss carryforwards and certain other tax attributes are now subject to limitations.
If a corporation undergoes an “ownership change” within the meaning of Section 382 of the U.S. Internal Revenue Code, the corporation’s net operating loss carryforwards and certain other tax attributes arising from before the ownership change are subject to limitations on use after the ownership change. In general, an ownership change occurs if there is a cumulative change in the corporation’s equity ownership by certain stockholders that exceeds fifty percentage points over a rolling three-year period. Similar rules may apply under state tax laws. Our 2017 merger involving OncoGenex and Achieve Life Sciences, Inc. resulted in an ownership change for OncoGenex and, accordingly, OncoGenex’s net operating loss carryforwards and certain other tax attributes will be subject to limitations on their use after the merger. Additional ownership changes in the future could result in additional limitations on the combined organization’s net operating loss carryforwards. Consequently, even if we achieve profitability, we may not be able to utilize a material portion of our net operating loss carryforwards and other tax attributes, which could have a material adverse effect on cash flow and results of operations.
If equity research analysts do not publish research or reports, or publish unfavorable research or reports, about us, our business, or our market, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that equity research analysts publish about us and our business. We do not have any control over the equity research analysts that provide research coverage of our common stock or the content and opinions included in their reports. The price of our stock could decline if one or more equity research analysts downgrades our stock or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our stock could decrease, which in turn could cause our stock price or trading volume to decline.
58
General Risk Factors
We are at risk of securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities, including in circumstances where such declines occur in close proximity to the announcement of clinical trial results. Additionally, our stock price and those of other biotechnology and biopharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We incur costs and demands upon management as a result of complying with the laws and regulations affecting public companies.
We incur significant legal, accounting and other expenses associated with public company reporting requirements. We also incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act, as well as rules implemented by the SEC and The Nasdaq Capital Market. These rules and regulations impose significant legal and financial compliance costs and make some activities more time-consuming and costly. In addition, it may be difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers, which may adversely affect investor confidence and could cause our business or stock price to suffer.
If we raise additional capital, the terms of the financing transactions may cause dilution to existing stockholders or contain terms that are not favorable to us.
In the future, we plan to raise additional capital through private placements or public offerings of our equity or debt securities. We cannot be certain that additional funding will be available on acceptable terms, if at all. To the extent that we raise additional financing by issuing equity securities, we may do so at a price per share that represents a discount to the then-current per share trading price of our common stock and our stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants, such as limitations on our ability to incur additional indebtedness, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely affect our ability to conduct our business.
Shareholder activists could cause a disruption to our business.
An activist investor may indicate disagreement with our strategic direction or capital allocation policies and may seek representation on our board of directors. Our business, operating results or financial condition could be adversely affected and may result in, among other things:
Anti-takeover provisions under Delaware law could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our management.
Because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporate Law, which prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. Although we believe these provisions collectively will provide for an opportunity to receive higher bids by requiring potential acquirors to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove then current management by making it more difficult for stockholders to replace members of the board of directors, which is responsible for appointing the members of management.
Our bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our bylaws provide that the Court of Chancery of the State of Delaware is the sole and exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty owed by any of our directors, officers or other
59
employees to us or our stockholders, any action asserting a claim against us arising pursuant to any provisions of the Delaware General Corporation Law, our certificate of incorporation or our bylaws, or any action asserting a claim against us that is governed by the internal affairs doctrine. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. If a court were to find the choice of forum provision contained in the bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions.
We are a smaller reporting company and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are currently a “smaller reporting company” as defined in the Exchange Act, and are thus allowed to provide simplified executive compensation disclosures in our filings, are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that an independent registered public accounting firm provide an attestation report on the effectiveness of internal control over financial reporting and have certain other decreased disclosure obligations in our SEC filings. We cannot predict whether investors will find our common stock less attractive because of our reliance on any of these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
U.S. federal tax reform and changes in other tax laws could increase our tax burden and adversely affect our business and financial condition.
In December 2017, the U.S. government enacted comprehensive tax legislation, the Tax Cuts and Jobs Act of 2017, significantly reforming the Internal Revenue Code of 1986, as amended. These changes include, among others, (i) a permanent reduction to the corporate income tax rate, (ii) a partial limitation on the deductibility of business interest expense, (iii) a shift of the U.S. taxation of multinational corporations from a tax on worldwide income to a territorial system (along with certain rules designed to prevent erosion of the U.S. income tax base) and (iv) a one-time tax on accumulated offshore earnings held in cash and illiquid assets, with the latter taxed at a lower rate.
In addition, beginning in 2022, the recently enacted tax legislation will require research and experimental expenditures to be capitalized and amortized ratably over a five-year period. Any such expenditures attributable to research conducted outside the United States must be capitalized and amortized over a 15-year period.
Notwithstanding the reduction in the corporate income tax rate, the overall impact of this tax reform is uncertain, and our business and financial condition could be adversely affected. Furthermore, it is uncertain if and to what extent various states will conform to the enacted federal tax law or any newly enacted federal legislation. In addition, new legislation or regulation which could affect our tax burden could be enacted by any governmental authority. We cannot predict the timing or extent of such tax‑related developments which could have a negative impact on our financial results. Additionally, we use our best judgment in attempting to quantify and reserve for these tax obligations. However, a challenge by a taxing authority, our ability to utilize tax benefits such as carryforwards or tax credits, or a deviation from other tax‑related assumptions could have a material adverse effect on our business, results of operations, or financial condition.
60
Item 6. Exhibits
Exhibit |
|
Description |
|
|
|
10.1 |
|
|
|
|
|
10.2 |
|
|
|
|
|
31.1 |
|
|
|
|
|
32.1# |
|
|
|
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document |
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document
|
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
|
|
# The certifications attached as Exhibits 32.1 and 32.2 accompany this Quarterly Report on Form 10-Q pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
61
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
ACHIEVE LIFE SCIENCES, INC. |
|
|
|
|
|
Date: May 9, 2023 |
|
By: |
/s/ John Bencich |
|
|
|
John Bencich |
|
|
|
Chief Executive Officer (Principal Executive and Financial Officer) |
62