FWP: Filing under Securities Act Rules 163/433 of free writing prospectuses
Published on December 2, 2019

Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration Statement No. 333-234530 December 2, 2019 ACHIEVE Life Sciences NASDAQ:ACHV Corporate Presentation December 2019 1

Forward Looking Statements ACHIEVE Life Sciences This presentation contains forward-looking statements, including, but not limited to, statements regarding the timing of planned clinical development activities of cytisinicline; the projected path toward potential regulatory approval; the safety, efficacy and commercial potential of cytisinicline; the potential market for cytisinicline; the benefits of cytisinicline relative to competitors; the anticipated benefits of cytisinicline; plans, objectives, expectations and intentions with respect to future operations. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Achieve Life Sciences, Inc. (“we,” “us,” “our,” or “the Company”) may not actually achieve its plans or product development goals in a timely manner, if at all, or otherwise carry out the intentions or meet the expectations or projections disclosed in these forward-looking statements. These statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward-looking statements, including, among others, general business and economic conditions; the need for and ability to obtain additional financing; the risk that cytisinicline may not demonstrate the hypothesized or expected benefits; the risk that cytisinicline will not receive regulatory approval or be successfully commercialized; the risk that new developments in the smoking cessation landscape require changes in business strategy or clinical development plans; the risk that the Company's intellectual property may not be adequately protected; other risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates that are safe and effective for use as human therapeutics; and the other factors described in the risk factors set forth in the Company's filings with the Securities and Exchange Commission from time to time, including its Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q. The Company undertakes no obligation to update the forward-looking statements contained herein or to reflect events or circumstances occurring after the date hereof, other than as may be required by applicable law. 2

Free Writing Prospectus ACHIEVE Life Sciences This presentation highlights basic information about us and the offering. Because it is a summary that has been prepared solely for informational purposes, it does not contain all of the information that you should consider before investing in our company. Except as otherwise indicated, this presentation speaks only as of the date hereof This presentation does not constitute an offer to sell, nor a solicitation of an offer to buy, any securities by any person in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation. Neither the Securities and Exchange Commission (the “SEC”) nor any other regulatory body has approved or disapproved of our securities or passed upon the accuracy or adequacy of this presentation. Any representation to the contrary is a criminal offense. This presentation includes industry and market data that we obtained from industry publications and journals, third-party studies and surveys, internal company studies and surveys, and other publicly available information. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. In addition, we do not know all of the assumptions that were used in preparing the forecasts from the sources relied upon or cited herein. We have filed a Registration Statement on Form S-1 with the SEC, including a preliminary prospectus dated December 2, 2019 (the “Preliminary Prospectus”) with respect to the offering of our securities to which this communication relates. Before you invest, you should read the Preliminary Prospectus (including the risk factors described therein) and, when available, the final prospectus relating to the offering, and the other documents filed with the SEC and incorporated by reference into the Preliminary Prospectus, for more complete information about us and the offering. You may obtain these documents, including the Preliminary Prospectus, for free by visiting EDGAR on the SEC website at http://sec.gov. Alternatively, we or any underwriter participating in the offering will arrange to send you the prospectus if you request it by contacting Ladenburg Thalmann & Co. Inc., 277 Park Avenue, 26th Floor, New York, NY 10172 or by email at prospectus@ladenburg.com. 3

Corporate Overview 4

Cytisinicline: A Potential New Treatment for Millions of Smokers ACHIEVE Life Sciences Achieve acquired the global rights* to cytisinicline from Sopharma AD Exclusively focused on the development and commercialization of cytisinicline for smoking cessation & nicotine addiction Robust Historical Data More than 10,000 participants in cytisinicline clinical trials to date Completed 2 investigator-led Phase 3 clinical trials in over 2,000 patients Over 20 years of in-market experience in over 20 million patients under brand name TABEX® Over 15 million cases in European safety database Strong Execution NIH partnership to complete IND enabling studies Completed Phase 1/2 repeat-dose PK/PD study Phase 2b ORCA-1 trial completed in Q2 2019 showing statistically significant quit rates (N=254) Phase 3 trial designs and NDA plans already reviewed with FDA * Excluding certain countries in Central and Eastern Europe, Scandinavia, North Africa, the Middle East and Central Asia, as well as Vietnam [Bar Chat] [Bar Chat] 5
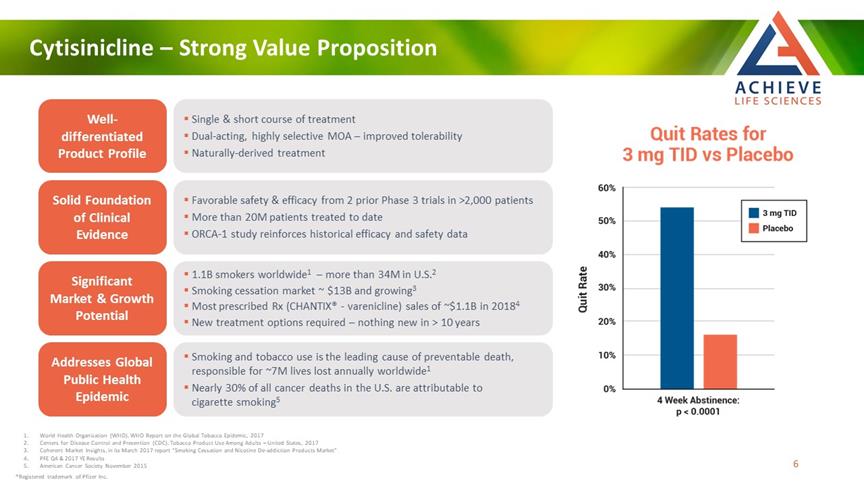
Cytisinicline – Strong Value Proposition ACHIEVE Life Sciences Well-differentiated Product Profile Solid Foundation of Clinical Evidence Significant Market & Growth Potential Addresses Global Public Health Epidemic Single & short course of treatment Dual-acting, highly selective MOA – improved tolerability Naturally-derived treatment Favorable safety & efficacy from 2 prior Phase 3 trials in >2,000 patients More than 20M patients treated to date ORCA-1 study reinforces historical efficacy and safety data 1.1B smokers worldwide1 – more than 34M in U.S.2 Smoking cessation market ~ $13B and growing3 Most prescribed Rx (CHANTIX® - varenicline) sales of ~$1.1B in 20184 New treatment options required – nothing new in > 10 years Smoking and tobacco use is the leading cause of preventable death, responsible for ~7M lives lost annually worldwide1 Nearly 30% of all cancer deaths in the U.S. are attributable to cigarette smoking5 World Health Organization (WHO). WHO Report on the Global Tobacco Epidemic, 2017 Centers for Disease Control and Prevention (CDC). Tobacco Product Use Among Adults – United States, 2017 Coherent Market Insights, in its March 2017 report “Smoking Cessation and Nicotine De-addiction Products Market” PFE Q4 & 2017 YE Results American Cancer Society November 2015 ®Registered trademark of Pfizer Inc. [Bar Chat] 6
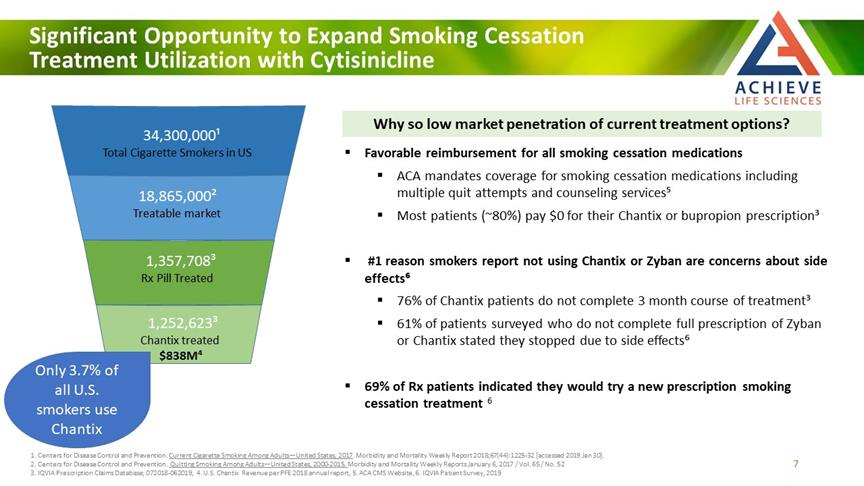
Significant Opportunity to Expand Smoking Cessation Treatment Utilization with Cytisinicline ACHIEVE Life Sciences Why so low market penetration of current treatment options? Favorable reimbursement for all smoking cessation medications ACA mandates coverage for smoking cessation medications including multiple quit attempts and counseling services⁵Most patients (~80%) pay $0 for their Chantix or bupropion prescription³ #1 reason smokers report not using Chantix or Zyban are concerns about side effects⁶ 76% of Chantix patients do not complete 3 month course of treatment³ 61% of patients surveyed who do not complete full prescription of Zyban or Chantix stated they stopped due to side effects⁶ 69% of Rx patients indicated they would try a new prescription smoking cessation treatment 6 34,300,000¹ Total Cigarette Smokers in US 18,865,000 Treatable market 1,357,708³ Rx Pill Treated 1,252,623³ Chantix treated $838M⁴ Only 3.7% of all U.S. smokers use Chantix 1. Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults—United States, 2017. Morbidity and Mortality Weekly Report 2018;67(44):1225-32 [accessed 2019 Jan 30]. 2. Centers for Disease Control and Prevention. Quitting Smoking Among Adults—United States, 2000-2015. Morbidity and Mortality Weekly Reports January 6, 2017 / Vol. 65 / No. 52 3. IQVIA Prescription Claims Database; 072018-062019, 4. U.S. Chantix Revenue per PFE 2018 annual report, 5. ACA CMS Website, 6. IQVIA Patient Survey, 2019 7
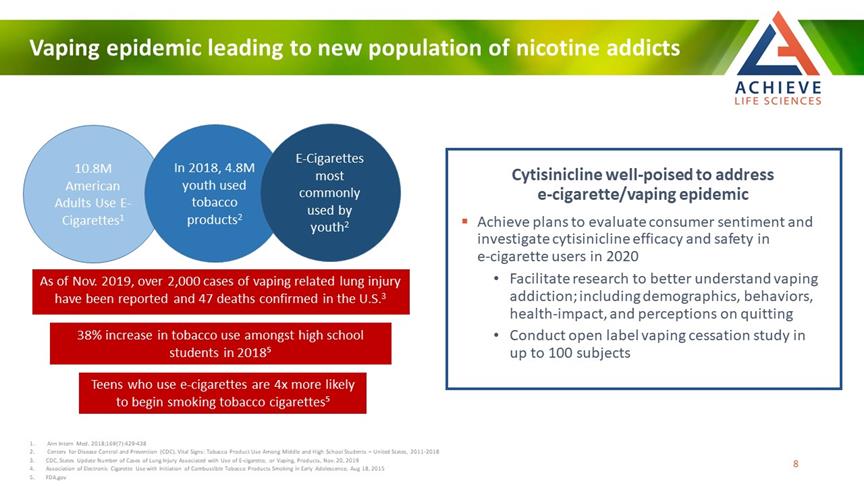
Vaping epidemic leading to new population of nicotine addicts ACHIEVE Life Sciences 10.8M American Adults Use E-Cigarettes1 In 2018, 4.8M youth used tobacco products2 E-Cigarettes most commonly used by youth2 As of Nov. 2019, over 2,000 cases of vaping related lung injury have been reported and 47 deaths confirmed in the U.S.3 38% increase in tobacco use amongst high school students in 2018⁵ Teens who use e-cigarettes are 4x more likely to begin smoking tobacco cigarettes⁵ Cytisinicline well-poised to address e-cigarette/vaping epidemic Achieve plans to evaluate consumer sentiment and investigate cytisinicline efficacy and safety in e-cigarette users in 2020 Facilitate research to better understand vaping addiction; including demographics, behaviors, health-impact, and perceptions on quitting 1 Conduct open label vaping cessation study in up to 100 subjects Ann Intern Med. 2018;169(7):429-438 2 Centers for Disease Control and Prevention (CDC). Vital Signs: Tobacco Product Use Among Middle and High School Students – United States, 2011-2018 3 CDC, States Update Number of Cases of Lung Injury Associated with Use of E-cigarette, or Vaping, Products, Nov. 20, 2019 4 Association of Electronic Cigarette Use with Initiation of Combustible Tobacco Products Smoking in Early Adolescence, Aug 18, 2015 FDA.gov 8

FDA Failed Crackdown on Nicotine ACHIEVE Life Sciences In July 2017, the FDA announced plans to cut the level of nicotine allowed in cigarettes¹ In November 2019, the FDA abandoned the plan to slash cigarette nicotine levels More people in the U.S. are addicted to nicotine than to any other drug² There are approximately 50 million people in the U.S. who are addicted to some type of tobacco product2 1 FDA.gov https://www.fda.gov/tobacco-products/public-health-education/how-could-lowering-nicotine-levels-cigarettes-change-future-public-health 2 American Society of Addiction Medicine. Public Policy Statement on Nicotine Addiction and Tobaccoexternal icon. Chevy Chase (MD): American Society of Addiction Medicine, 2008 [accessed 2017 Jan 24] 3 “Understanding Nicotine”; www.addictioncenter.com/nicotine [Bar Chat] [Bar Chat] [Bar Chat] 9
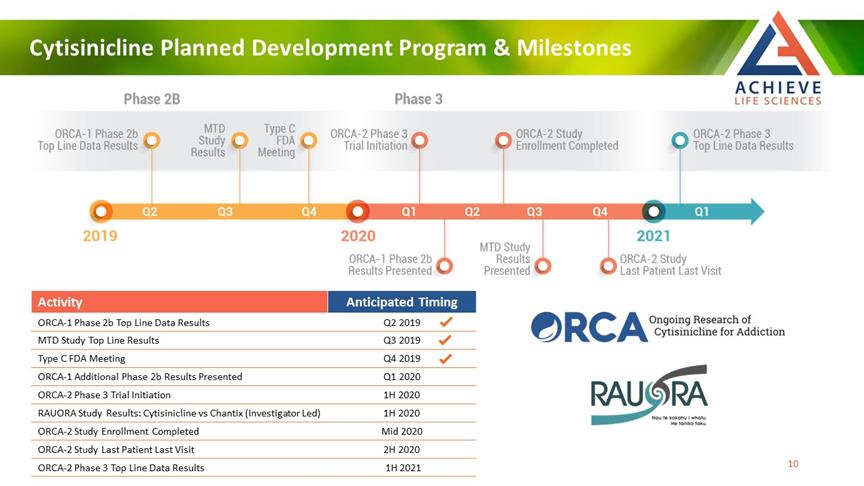
Cytisinicline Planned Development Program & Milestones ACHIEVE Life Sciences Activity Anticipated Timing ORCA-1 Phase 2b Top Line Data Results Q2 2019 MTD Study Top Line Results Q3 2019 Type C FDA Meeting Q4 2019 ORCA-1 Additional Phase 2b Results Presented Q1 2020 ORCA-2 Phase 3 Trial Initiation 1H 2020 RAUORA Study Results: Cytisinicline vs Chantix (Investigator Led) 1H 2020 ORCA-2 Study Enrollment Completed Mid 2020 ORCA-2 Study Last Patient Last Visit 2H 2020 ORCA-2 Phase 3 Top Line Data Results 1H 2021 [Bar Char] [Bar Char] [Bar Char] 10
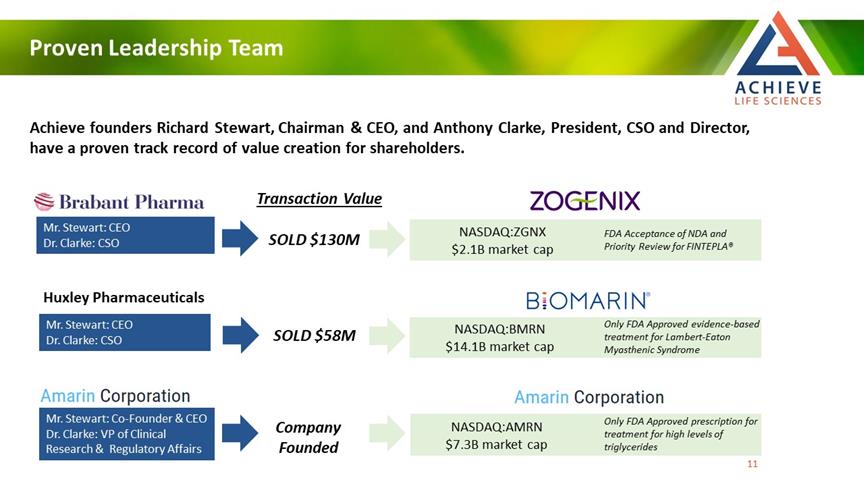
Proven Leadership Team ACHIEVE Life Sciences Achieve founders Richard Stewart, Chairman & CEO, and Anthony Clarke, President, CSO and Director, have a proven track record of value creation for shareholders. Mr. Stewart: CEO Dr. Clarke: CSO Huxley Pharmaceuticals Mr. Stewart: CEO Dr. Clarke: CSO Amarin Corporation Mr. Stewart: Co-Founder & CEO Dr. Clarke: VP of Clinical Research & Regulatory Affairs Transaction Value SOLD $130M SOLD $58M Company Founded NASDAQ:ZGNX $2.1B market cap FDA Acceptance of NDA and Priority Review for FINTEPLA® NASDAQ:BMRN $14.1B market cap Only FDA Approved evidence-based treatment for Lambert-Eaton Myasthenic Syndrome Amarin Corporation NASDAQ:AMRN $7.3B market cap Only FDA Approved prescription for treatment for high levels of triglycerides [Bar Char] [Bar Char] [Bar Char] 11

The Cytisinicline Difference Dual-Acting, Highly-Targeted MOA Single & Short Treatment Very Well-Tolerated 12
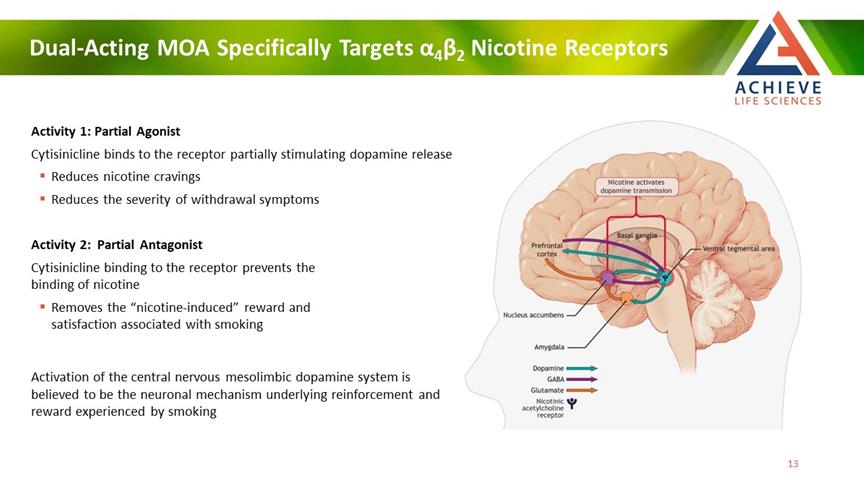
Dual-Acting MOA Specifically Targets α4β2 Nicotine Receptors ACHIEVE Life Sciences Activity 1: Partial Agonist Cytisinicline binds to the receptor partially stimulating dopamine release Reduces nicotine cravings Reduces the severity of withdrawal symptoms Activity 2: Partial Antagonist Cytisinicline binding to the receptor prevents the binding of nicotine Removes the “nicotine-induced” reward and satisfaction associated with smoking Activation of the central nervous mesolimbic dopamine system is believed to be the neuronal mechanism underlying reinforcement and reward experienced by smoking 13
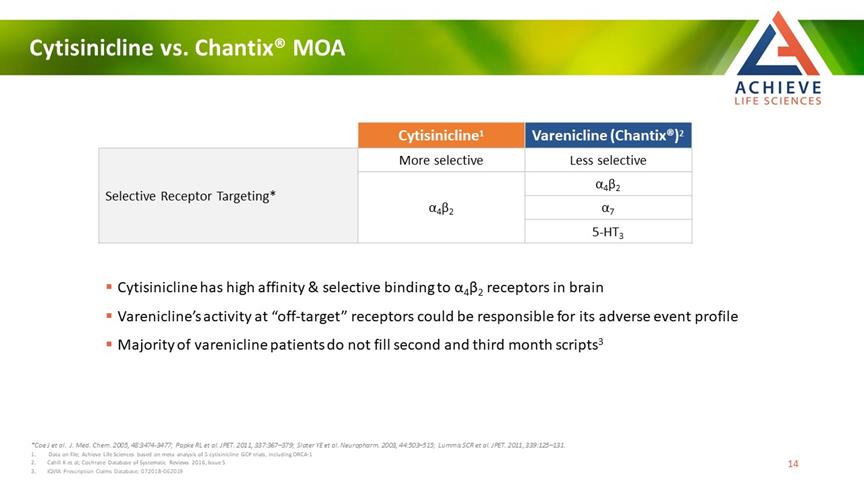
Cytisinicline vs. Chantix® MOA ACHIEVE Life Sciences Cytisinicline1 Varenicline (Chantix®)2 More selective Less selective Selective Receptor Targeting* α4β2 α4β2 α7 5-HT3 Cytisinicline has high affinity & selective binding to α4β2 receptors in brain Varenicline’s activity at “off-target” receptors could be responsible for its adverse event profile Majority of varenicline patients do not fill second and third month scripts3 *Coe J et al. J. Med. Chem. 2005, 48:3474-3477; Papke RL et al. JPET. 2011, 337:367–379; Slater YE et al. Neuropharm. 2003, 44:503–515; Lummis SCR et al. JPET. 2011, 339:125–131. 1 Data on file; Achieve Life Sciences based on meta analysis of 5 cytisinicline GCP trials, including ORCA-1 2 Cahill K et al; Cochrane Database of Systematic Reviews 2016, Issue 5 3 IQVIA Prescription Claims Database; 072018-062019 14
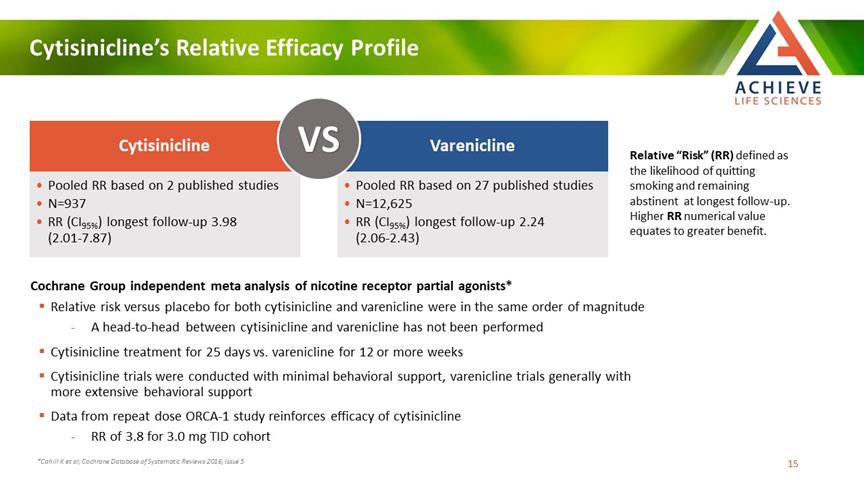
Cytisinicline’s Relative Efficacy Profile ACHIEVE Life Sciences Cytisinicline vs Varenicline Pooled RR based on 2 published studies N=937 RR (CI95%) longest follow-up 3.98 (2.01-7.87) Pooled RR based on 27 published studies N=12,625 RR (CI95%) longest follow-up 2.24 (2.06-2.43) Relative “Risk” (RR) defined as the likelihood of quitting smoking and remaining abstinent at longest follow-up. Higher RR numerical value equates to greater benefit. Cochrane Group independent meta analysis of nicotine receptor partial agonists* Relative risk versus placebo for both cytisinicline and varenicline were in the same order of magnitude A head-to-head between cytisinicline and varenicline has not been performed Cytisinicline treatment for 25 days vs. varenicline for 12 or more weeks Cytisinicline trials were conducted with minimal behavioral support, varenicline trials generally with more extensive behavioral support Data from repeat dose ORCA-1 study reinforces efficacy of cytisinicline RR of 3.8 for 3.0 mg TID cohort *Cahill K et al; Cochrane Database of Systematic Reviews 2016, Issue 5 15
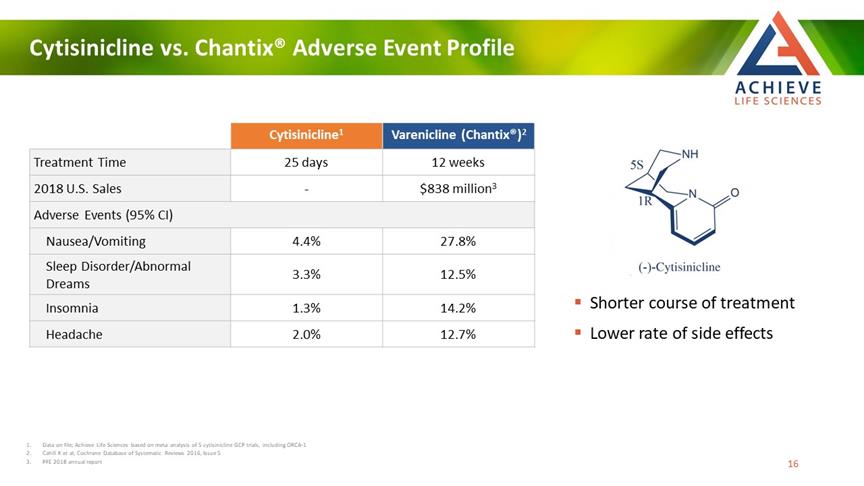
Cytisinicline vs. Chantix® Adverse Event Profile ACHIEVE Life Sciences Cytisinicline1 Varenicline (Chantix®)2 Treatment Time 25 days 12 weeks 2018 U.S. Sales -$838 million3 Adverse Events (95% CI) Nausea/Vomiting 4.4% 27.8% Sleep Disorder/Abnormal Dreams 3.3% 12.5% Insomnia 1.3% 14.2% Headache 2.0% 12.7% Shorter course of treatment Lower rate of side effects 1 Data on file; Achieve Life Sciences based on meta analysis of 5 cytisinicline GCP trials, including ORCA-1 2 Cahill K et al; Cochrane Database of Systematic Reviews 2016, Issue 5 3 PFE 2018 annual report [Bar Chat] 16

Cytisinicline vs. Chantix® - RAUORA Study ACHIEVE Life Sciences The RAUORA Study is a single investigator led study in New Zealand comparing Tabex (contains cytisine) and Champix® 2,140 patient randomized study Primary outcome is CO verified continuous abstinence at 6 months post-quite date 12 week treatment period RAUORA is funded by the Health Research Council of New Zealand Results are expected Q1-2020 [Bar Chat] [Bar Chat] [Bar Chat] 17

Cytisinicline Clinical Development 18
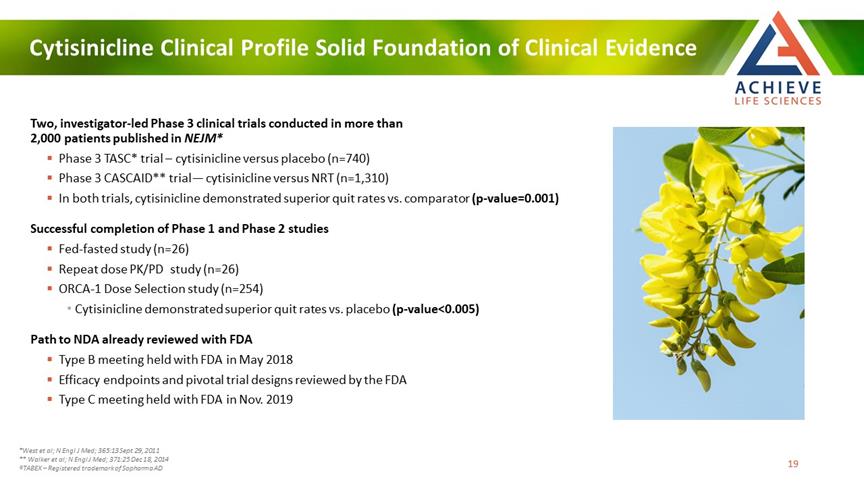
Cytisinicline Clinical Profile Solid Foundation of Clinical Evidence ACHIEVE Life Sciences Two, investigator-led Phase 3 clinical trials conducted in more than 2,000 patients published in NEJM* Phase 3 TASC* trial – cytisinicline versus placebo (n=740)Phase 3 CASCAID** trial— cytisinicline versus NRT (n=1,310) In both trials, cytisinicline demonstrated superior quit rates vs. comparator (p-value=0.001) Successful completion of Phase 1 and Phase 2 studies Fed-fasted study (n=26)Repeat dose PK/PD study (n=26) ORCA-1 Dose Selection study (n=254) Cytisinicline demonstrated superior quit rates vs. placebo (p-value<0.005) Path to NDA already reviewed with FDA Type B meeting held with FDA in May 2018 Efficacy endpoints and pivotal trial designs reviewed by the FDA Type C meeting held with FDA in Nov. 2019 *West et al; N Engl J Med; 365:13 Sept 29, 2011 ** Walker et al; N Engl J Med; 371:25 Dec 18, 2014 ®TABEX – Registered trademark of Sopharma AD [Bar Chat] 19
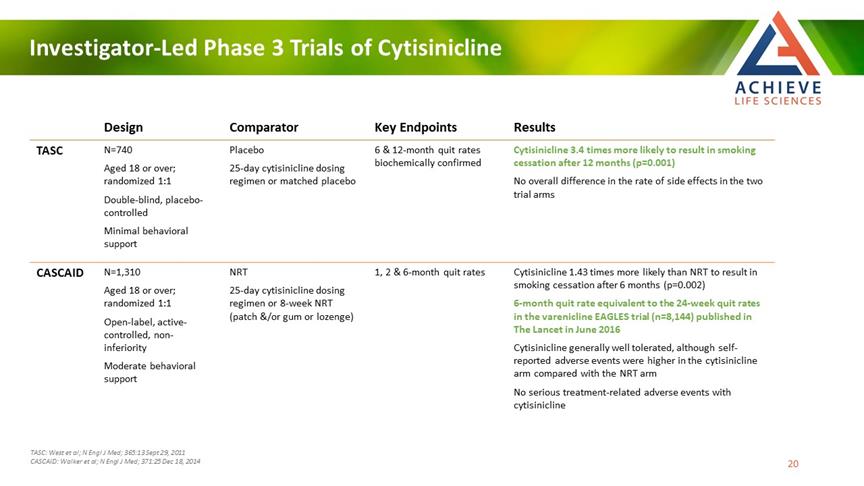
Investigator-Led Phase 3 Trials of Cytisinicline ACHIEVE Life Sciences Design Comparator Key Endpoints Results TASC N=740 Aged 18 or over; randomized 1:1 Double-blind, placebo-controlled Minimal behavioral support Placebo 25-day cytisinicline dosing regimen or matched placebo 6 & 12-month quit rates biochemically confirmed Cytisinicline 3.4 times more likely to result in smoking cessation after 12 months (p=0.001) No overall difference in the rate of side effects in the two trial arms CASCAID N=1,310 Aged 18 or over; randomized 1:1 Open-label, active-controlled, non-inferiority Moderate behavioral support NRT 25-day cytisinicline dosing regimen or 8-week NRT (patch &/or gum or lozenge) 1, 2 & 6-month quit rates Cytisinicline 1.43 times more likely than NRT to result in smoking cessation after 6 months (p=0.002) 6-month quit rate equivalent to the 24-week quit rates in the varenicline EAGLES trial (n=8,144) published in The Lancet in June 2016 Cytisinicline generally well tolerated, although self-reported adverse events were higher in the cytisinicline arm compared with the NRT arm No serious treatment-related adverse events with cytisinicline TASC: West et al; N Engl J Med; 365:13 Sept 29, 2011 CASCAID: Walker et al; N Engl J Med; 371:25 Dec 18, 2014 20
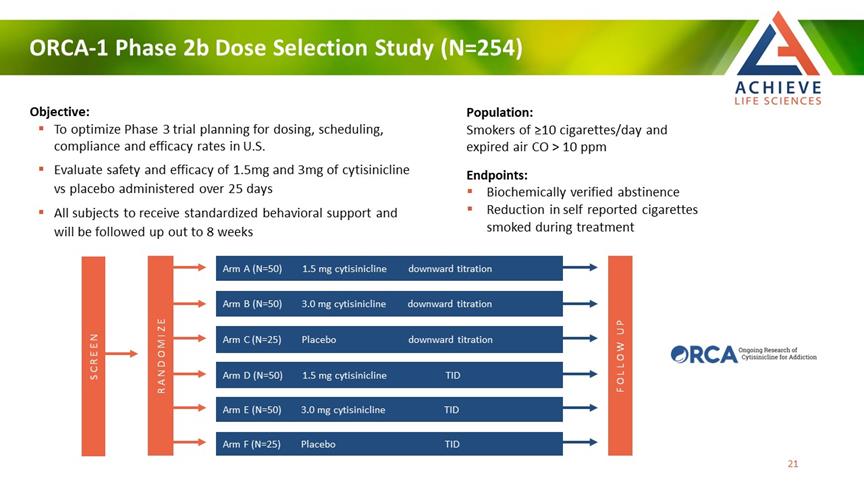
ORCA-1 Phase 2b Dose Selection Study (N=254) ACHIEVE Life Sciences Objective: To optimize Phase 3 trial planning for dosing, scheduling, compliance and efficacy rates in U.S. Evaluate safety and efficacy of 1.5mg and 3mg of cytisinicline vs placebo administered over 25 days All subjects to receive standardized behavioral support and will be followed up out to 8 weeks Population: Smokers of ≥10 cigarettes/day and expired air CO > 10 ppm Endpoints: Biochemically verified abstinence Reduction in self reported cigarettes smoked during treatment Randomize Screen Arm A (N=50) 1.5 mg cytisinicline downward titration Arm B (N=50) 3.0 mg cytisinicline downward titration Arm C (N=25) Placebo downward titration Arm D (N=50) 1.5 mg cytisinicline TID Arm E (N=50) 3.0 mg cytisinicline TID Arm F (N=25) Placebo Tid Follow Up [Bar Chat] 21
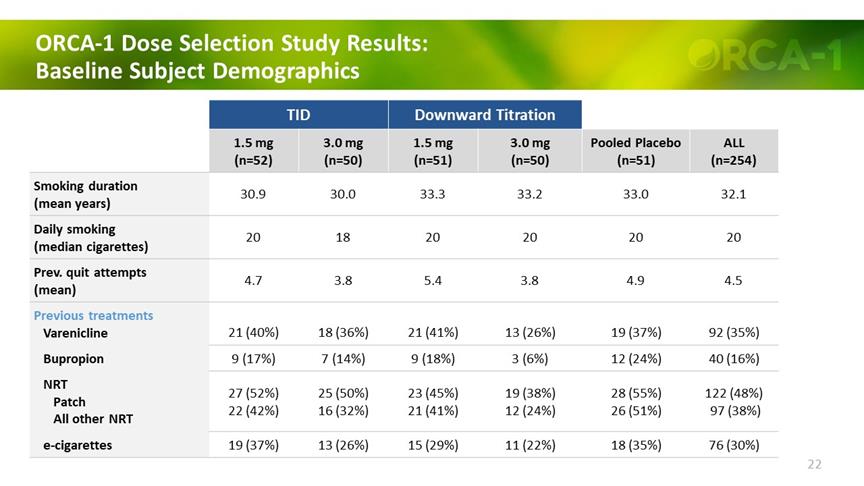
ORCA-1 Dose Selection Study Results: Baseline Subject Demographics TID Downward Titration 1.5 mg (n=52) 3.0 mg (n=50) 1.5 mg (n=51) 3.0 mg (n=50) Pooled Placebo (n=51) ALL (n=254) Smoking duration (mean years) 30.9 30.0 33.3 33.2 33.0 32.1 Daily smoking (median cigarettes) 20 18 20 20 20 20 Prev. quit attempts (mean) 4.7 3.8 5.4 3.8 4.9 4.5 Previous treatments Varenicline 21 (40%) 18 (36%) 21 (41%) 13 (26%) 19 (37%) 92 (35%) Bupropion 9 (17%) 7 (14%) 9 (18%) 3 (6%) 12 (24%) 40 (16%) NRT Patch All other NRT 27 (52%) 22 (42%) 25 (50%) 16 (32%) 23 (45%) 21 (41%) 19 (38%) 12 (24%) 28 (55%) 26 (51%) 122 (48%) 97 (38%) e-cigarettes 19 (37%) 13 (26%) 15 (29%) 11 (22%) 18 (35%) 76 (30%) 22
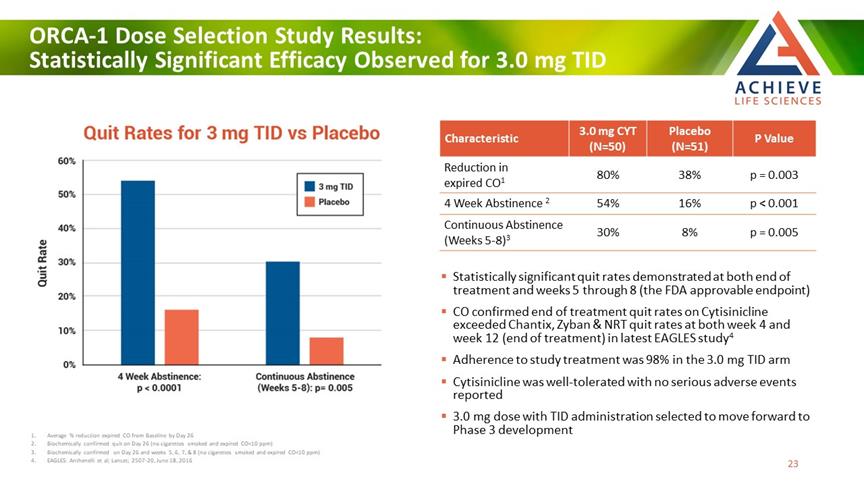
ORCA-1 Dose Selection Study Results: Statistically Significant Efficacy Observed for 3.0 mg TID ACHIEVE Life Sciences Characteristic 3.0 mg CYT (N=50) Placebo (N=51) P Value Reduction in expired CO1 80% 38% p = 0.003 4 Week Abstinence 2 54% 16% p < 0.001 Continuous Abstinence (Weeks 5-8)3 30% 8% p = 0.005 Statistically significant quit rates demonstrated at both end of treatment and weeks 5 through 8 (the FDA approvable endpoint) CO confirmed end of treatment quit rates on Cytisinicline exceeded Chantix, Zyban & NRT quit rates at both week 4 and week 12 (end of treatment) in latest EAGLES study4 Adherence to study treatment was 98% in the 3.0 mg TID arm Cytisinicline was well-tolerated with no serious adverse events reported 1 3.0 mg dose with TID administration selected to move forward to Phase 3 development Average % reduction expired CO from Baseline by Day 26 2 Biochemically confirmed quit on Day 26 (no cigarettes smoked and expired CO<10 ppm) 3 Biochemically confirmed on Day 26 and weeks 5, 6, 7, & 8 (no cigarettes smoked and expired CO<10 ppm) 4 EAGLES: Anthenelli et al; Lancet; 2507-20, June 18, 2016 [Bar Chat] 23
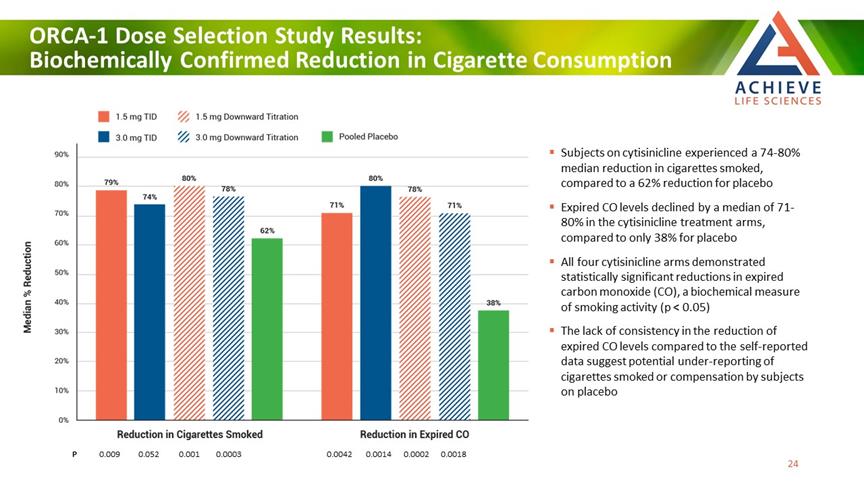
ORCA-1 Dose Selection Study Results: Biochemically Confirmed Reduction in Cigarette Consumption ACHIEVE Life Sciences Subjects on cytisinicline experienced a 74-80% median reduction in cigarettes smoked, compared to a 62% reduction for placebo Expired CO levels declined by a median of 71-80% in the cytisinicline treatment arms, compared to only 38% for placebo All four cytisinicline arms demonstrated statistically significant reductions in expired carbon monoxide (CO), a biochemical measure of smoking activity (p < 0.05) The lack of consistency in the reduction of expired CO levels compared to the self-reported data suggest potential under-reporting of cigarettes smoked or compensation by subjects on placebo P 0.009 0.052 0.001 0.0003 0.0042 0.0014 0.0002 0.0018 [Bar Chat] 24
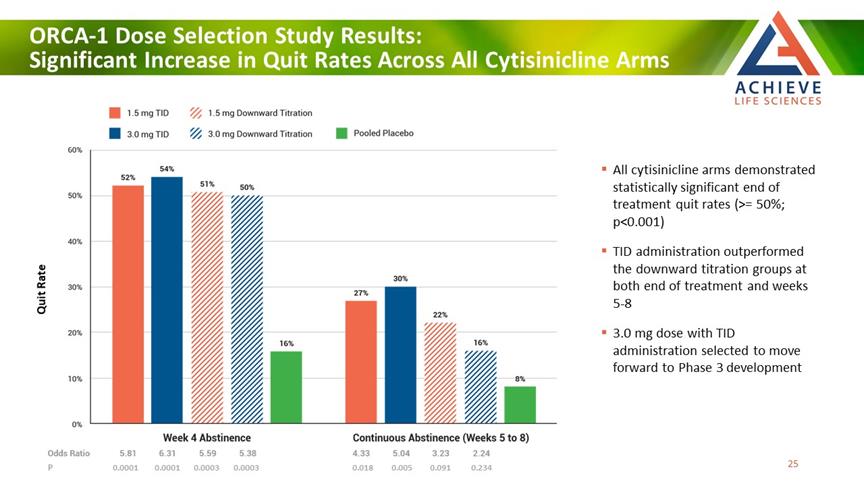
ORCA-1 Dose Selection Study Results: Significant Increase in Quit Rates Across All Cytisinicline Arms ACHIEVE Life Sciences All cytisinicline arms demonstrated statistically significant end of treatment quit rates (>= 50%; p<0.001) TID administration outperformed the downward titration groups at both end of treatment and weeks 5-8 3.0 mg dose with TID administration selected to move forward to Phase 3 development P 0.0001 0.0001 0.0003 0.0003 0.018 0.005 0.091 0.234 Quit Rate [Bar Chat] 25
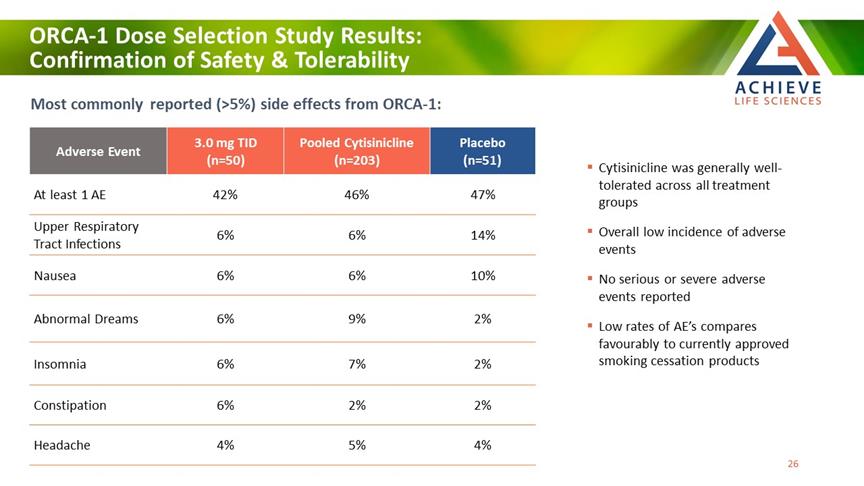
ORCA-1 Dose Selection Study Results: Confirmation of Safety & Tolerability ACHIEVE Life Sciences Most commonly reported (>5%) side effects from ORCA-1: Adverse Event 3.0 mg TID (n=50) Pooled Cytisinicline (n=203) Placebo (n=51) At least 1 AE 42% 46% 47% Upper Respiratory Tract Infections 6% 6% 14% Nausea 6% 6% 10% Abnormal Dreams 6% 9% 2% Insomnia 6% 7% 2% Constipation 6% 2% 2% Headache 4% 5% 4% Cytisinicline was generally well-tolerated across all treatment groups Overall low incidence of adverse events No serious or severe adverse events reported Low rates of AE’s compares favourably to currently approved smoking cessation products 26
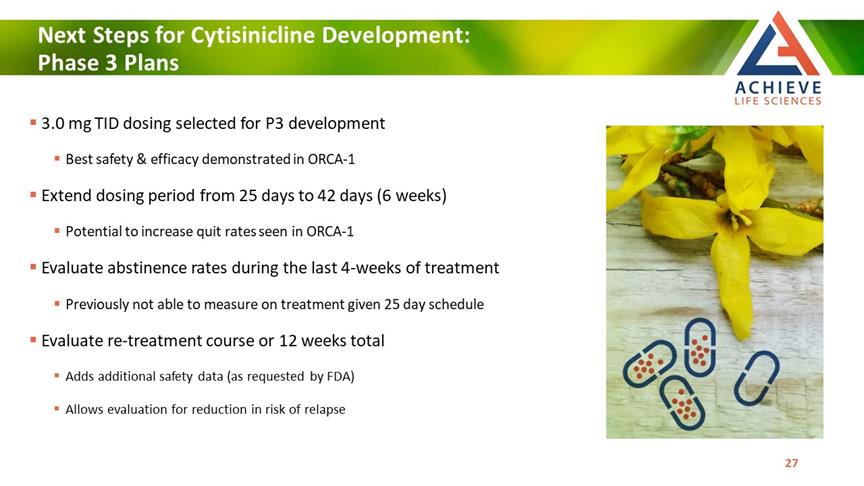
Next Steps for Cytisinicline Development: Phase 3 Plans ACHIEVE Life Sciences 3.0 mg TID dosing selected for P3 development Best safety & efficacy demonstrated in ORCA-1 Extend dosing period from 25 days to 42 days (6 weeks) Potential to increase quit rates seen in ORCA-1 Evaluate abstinence rates during the last 4-weeks of treatment Previously not able to measure on treatment given 25 day schedule Evaluate re-treatment course or 12 weeks total Adds additional safety data (as requested by FDA) Allows evaluation for reduction in risk of relapse [Bar Chat] 27
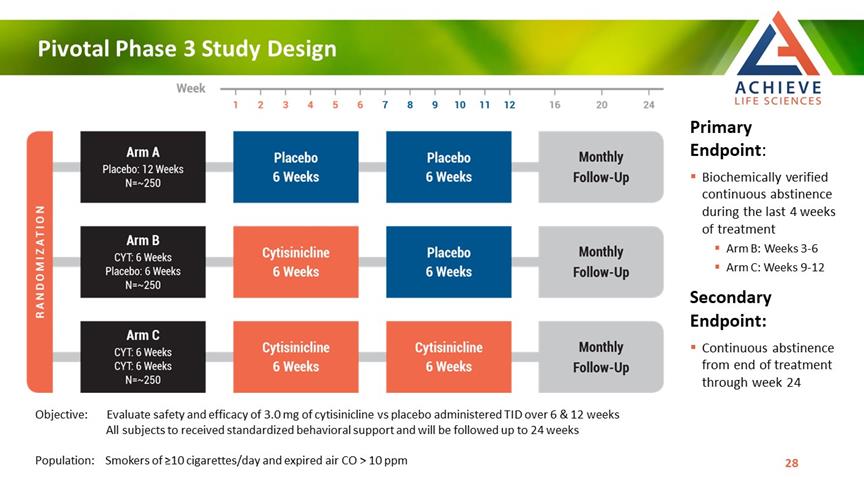
Pivotal Phase 3 Study Design ACHIEVE Life Sciences Primary Endpoint: Biochemically verified continuous abstinence during the last 4 weeks of treatment Arm B: Weeks 3-6 Arm C: Weeks 9-12 Secondary Endpoint: Continuous abstinence from end of treatment through week 24 Objective: Evaluate safety and efficacy of 3.0 mg of cytisinicline vs placebo administered TID over 6 & 12 weeks All subjects to received standardized behavioral support and will be followed up to 24 weeks Population: Smokers of ≥10 cigarettes/day and expired air CO > 10 ppm [Bar Chat] 28
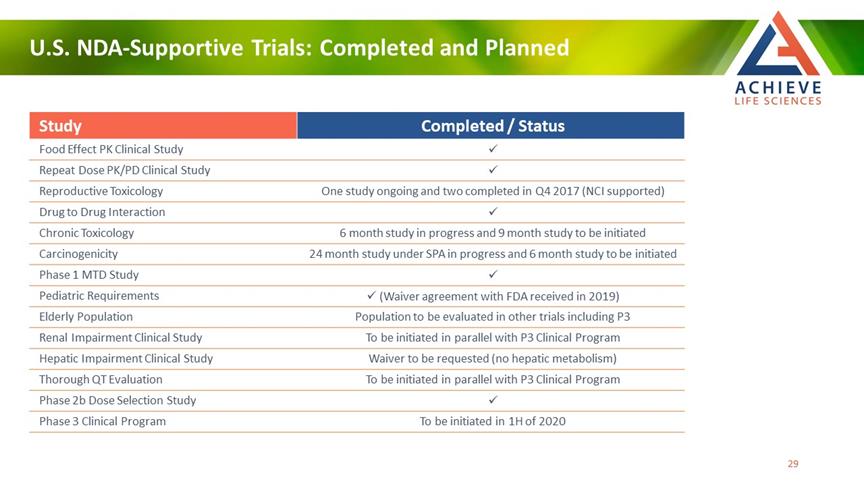
U.S. NDA-Supportive Trials: Completed and Planned ACHIEVE Life Sciences Study Completed / Status Food Effect PK Clinical Study ✓ Repeat Dose PK/PD Clinical Study ✓ Reproductive Toxicology One study ongoing and two completed in Q4 2017 (NCI supported) Drug to Drug Interaction ✓ Chronic Toxicology 6 month study in progress and 9 month study to be initiated Carcinogenicity 24 month study under SPA in progress and 6 month study to be initiated Phase 1 MTD Study ✓ Pediatric Requirements ✓ (Waiver agreement with FDA received in 2019) Elderly Population Population to be evaluated in other trials including P3 Renal Impairment Clinical Study To be initiated in parallel with P3 Clinical Program Hepatic Impairment Clinical Study Waiver to be requested (no hepatic metabolism) Thorough QT Evaluation To be initiated in parallel with P3 Clinical Program Phase 2b Dose Selection Study ✓ Phase 3 Clinical Program To be initiated in 1H of 2020 29

Market Opportunity 30
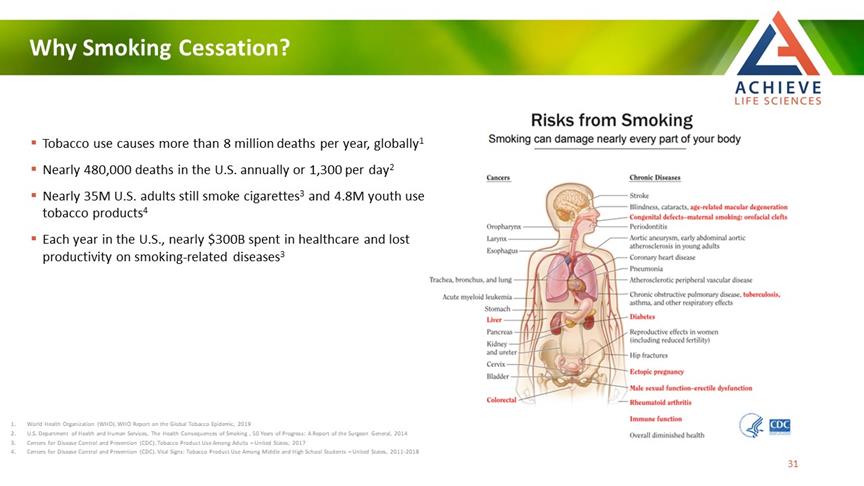
Why Smoking Cessation? ACHIEVE Life Sciences Tobacco use causes more than 8 million deaths per year, globally1 Nearly 480,000 deaths in the U.S. annually or 1,300 per day2 Nearly 35M U.S. adults still smoke cigarettes3 and 4.8M youth use tobacco products4 Each year in the U.S., nearly $300B spent in healthcare and lost productivity on smoking-related diseases3 1 World Health Organization (WHO). WHO Report on the Global Tobacco Epidemic, 2019 2 U.S. Department of Health and Human Services, The Health Consequences of Smoking, 50 Years of Progress: A Report of the Surgeon General, 2014 3 Centers for Disease Control and Prevention (CDC). Tobacco Product Use Among Adults – United States, 2017 4 Centers for Disease Control and Prevention (CDC). Vital Signs: Tobacco Product Use Among Middle and High School Students – United States, 2011-2018 Risks from smoking smoking can damage nearly every part of your body 31
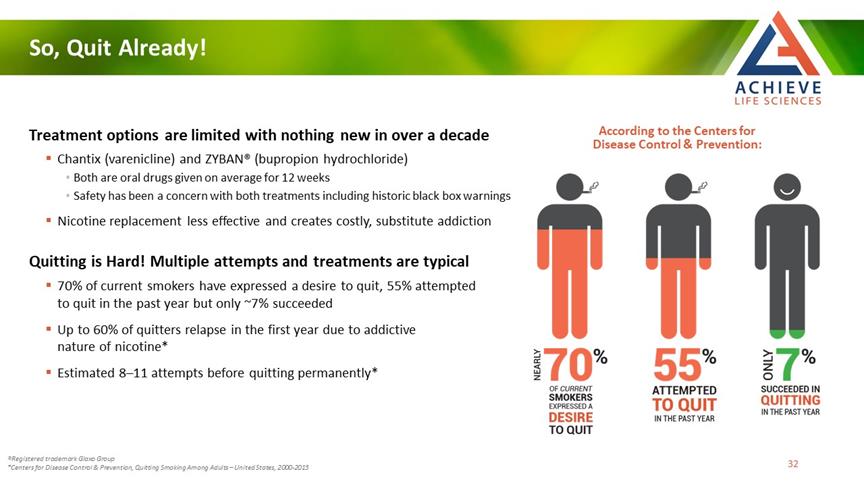
So, Quit Already! ACHIEVE LIFE SCIENCES Treatment options are limited with nothing new in over a decade Chantix (varenicline) and ZYBAN® (bupropion hydrochloride) Both are oral drugs given on average for 12 weeks Safety has been a concern with both treatments including historic black box warnings Nicotine replacement less effective and creates costly, substitute addiction Quitting is Hard! Multiple attempts and treatments are typical 70% of current smokers have expressed a desire to quit, 55% attempted to quit in the past year but only ~7% succeeded Up to 60% of quitters relapse in the first year due to addictive nature of nicotine* Estimated 8–11 attempts before quitting permanently* According to the Centers for Disease Control & Prevention: ®Registered trademark Glaxo Group *Centers for Disease Control & Prevention, Quitting Smoking Among Adults – United States, 2000-2015 NEARLY 70% OF CURRENT SMOKERS EXPRESSED A DESIRE TO QUIT 55% ATTEMPTED TO QUIT IN THE PAST YEAR ONLY 7% SUCCEEDED IN QUITTING IN THE PAST YEAT 32
Broad Product Protection ACHIEVE LIFE SCIENCES PATENT APPLICATIONS REGULATORY EXCLUSIVITY API SUPEXCLUSIVEPLY SECOND GENERATION CYTISINICLINE • Several patent families pursued globally including formulation, method of use, extraction • Issued patents – new cytisinicline salt formulation • Ongoing discovery and other development work in providing additional IP opportunities • Hatch-Waxman – 5 years for NCE • Europe – Up to 10 years in countries where cytisinicline is not already approved • Orange Book cytisinicline specification • Sopharma exclusive supply agreement to 4-6 year API lead time • 100% (-)- enantiomer of cytisinicline • Synthetic 100% (-)- cytisinicline not currently viable • Extraction know-how / trade secrets • University of Bristol exclusive license agreement • Next generation highly targeted cytisinicline derivatives for other indications 33

Capitalization 34
Capitalization ACHIEVE LIFE SCIENCES ▪ Cash, cash equivalents and investments of ~$7.4M as September 30, 2019 ▪ No debt ▪ Capitalization (as of Nov. 6, 2019): Common Shares Outstanding 8,352,764 Warrants (WAEP $4.12) 4,116,712 Outstanding under equity award plans 1,020,943 Fully Diluted Shares 13,490,419 35

Investment Highlights ACHIEVE LIFE SCIENCES Cytisinicline well-positioned to address global tobacco public health epidemic ▪ Addresses tobacco & nicotine addiction, leading cause of cancer and cardiovascular disease-related death ▪ Differentiation from currently available products, with history of black box warnings, has positive implications for improved safety and efficacy Large market opportunity and patient need ▪ ~$13B nicotine addiction market, with sales of leading product Chantix of >$1B in 2018* ▪ Solid foundation of clinical evidence with regards to both safety and efficacy ▪ Two completed Phase 3 trials, with over 2,000 patients, supports safety and efficacy of cytisinicline ▪ Clear path to market ▪ Recent FDA interactions provide clear direction for NDA requirements in U.S. ▪ Proven management team ▪ Management team has a history of leading successful biopharma companies, including through acquisition *PFE Q4 & 2017 YE Results 36

ACHIEVE LIFE SCIENCES Thank You! 37

Achieve Management Team ACHIEVE LIFE SCIENCES Rick Stewart, Chairman & Chief Executive Officer John Bencich, MBA, EVP, Chief Financial & Operations Officer Anthony Clarke, PhD; Chief Scientific Officer Cindy Jacobs, PhD, MD; EVP, Chief Medical Officer Jaime Xinos, EVP, Commercial Nearly 25 years of experience in the pharmaceutical industry having founded and served as CEO for private and public companies Ricanto, Renown Pharma, Brabant Pharma, Huxley Pharma, and Amarin Corp. Also founded and held the positions of CFO and CBO of SkyePharma. An experienced financial executive with 20 years of experience in the pharmaceutical industry having served as CFO and in senior financial positions at multiple public and private companies including OncoGenex, Integrated Diagnostics, Allozyne, and Trubion (acquired by Emergent). Extensive experience in the biotechnology/pharmaceutical industry, Dr. Clarke is a founder and director of Ricanto, and currently serves as CSO of Renown Pharma. Dr. Clarke was CSO of Huxley Pharma, Brabant Pharma, and was VP Clinical Research and Regulatory Affairs of Amarin Corp. With over 30 years of experience in the biotechnology/pharmaceutical industry, Dr. Jacobs is an experienced executive in drug development. She served as EVP & CMO of OncoGenex, CMO & SVP of Corixa Corporation, and held VP Clinical Research positions at two other biopharmaceutical companies. Two decades of commercial experience, including VP, Marketing and Corporate Communications at OncoGenex, and previous marketing, commercial development, sales and marketing leadership roles at Pfizer, Novartis and Abbott Labs. 38
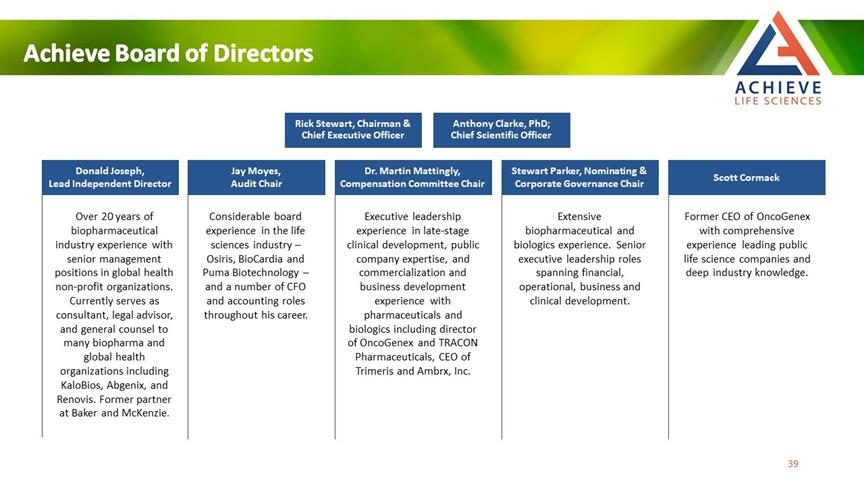
Achieve Board of Directors Rick Stewart, Chairman & Chief Executive Officer Anthony Clarke, PhD; Chief Scientific Officer Donald Joseph, Lead Independent Director Jay Moyes, Audit Chair Dr. Martin Mattingly, Compensation Committee Chair Stewart Parker, Nominating & Corporate Governance Chair Scott Cormack Over 20 years of biopharmaceutical industry experience with senior management positions in global health non-profit organizations. Currently serves as consultant, legal advisor, and general counsel to many biopharma and global health organizations including KaloBios, Abgenix, and Renovis. Former partner at Baker and McKenzie. Considerable board experience in the life sciences industry – Osiris, BioCardia and Puma Biotechnology – and a number of CFO and accounting roles throughout his career. Executive leadership experience in late-stage clinical development, public company expertise, and commercialization and business development experience with pharmaceuticals and biologics including director of OncoGenex and TRACON Pharmaceuticals, CEO of Trimeris and Ambrx, Inc. Extensive biopharmaceutical and biologics experience. Senior executive leadership roles spanning financial, operational, business and clinical development. Former CEO of OncoGenex with comprehensive experience leading public life science companies and deep industry knowledge. ACHIEVE LIFE SCIENCES 39