425: Filing under Securities Act Rule 425 of certain prospectuses and communications in connection with business combination transactions
Published on February 21, 2017
Filed by OncoGenex Pharmaceuticals, Inc.
Pursuant to Rule 425 under the Securities Act of 1933
Deemed filed pursuant to Rule 14a-12 of the Securities Exchange Act of 1934
Subject Company: OncoGenex Pharmaceuticals, Inc.
Commission File No.: 033-80623
On February 21, 2017, OncoGenex Pharmaceuticals, Inc. provided copies of the following posters to investors:
|
|
PACIFIC Trial: ARandomizedPhaseIIStudyofApatorsenandAbirateroneinPatientswithMetastaticCRPC
WhoHaveHadPSAProgressionWhileReceivingAbiraterone
Kim N. Chi1,2, Mark Fleming3, Katherine Sunderland1, Costantine Albany4, Joel Gingerich5, Fred Saad6, Scott North7, Alexander Starodub8, Richard Lauer9, Dean Ruether10, Madelaine Sgroi11, Mark Scholz12, Christopher Sweeney13
1BC Cancer Vancouver Centre, 2Vancouver Prostate Centre, 3Virginia Oncology Associates, 4Indiana University Melvin and Bren Simon Cancer Center, 5CancerCare Manitoba, 6Centre Hospitalier de l Université de Montréal (CHUM), 7Cross Cancer Institute, 8Goshen Center for Cancer Care, 9University of New Mexico Cancer Center, 10Alberta Health Services Tom Baker Cancer Centre, 11IU Health Central Indiana Cancer Centers, 12Prostate Oncology Specialists Inc, 13Dana-Farber Cancer Institute
BACKGROUND
Heat Shock Protein 27 (Hsp27)
Stress activated, ATP-independent member of the small heat shock protein group
Phospho-activated to form chaperoning oligomer which regulates multiple cell signaling and survival pathways
Inhibits apoptosis along intrinsic and extrinsic pathways
Involved in proteasome mediated degradation
Facilitates normal protein folding and function
Steroid receptors (AR, ER), growth factors (IGF-1, VEGF-1, FGF), cytokines (IL- 6, TGF-beta)
Highly expressed in many cancers including prostate cancer
High expression associated with poorer prognosis
Increases after castration therapy and in CRPC tissues
Bubendorf et al, J Natl Cancer Inst, 91:1758, 1999; Rocci et al, Cancer Res, 64:6595, 2004; Rocci et al, Cancer Res, 65:11083, 2005; Zoubeidi et al, Cancer Res, 67:10455, 2007
Apatorsen (OGX-427, OncoGenex Technologies Inc.)
Second generation phosphorothioate antisense oligonucleotide with 2-MOE modifications
Prolonged tissue half life ~10 days
Inhibits Hsp27 expression in vitro and in vivo with single agent anti-tumour activity
Enhances efficacy of hormone and chemotherapy
Disrupts AR function and enhances AR degradation
Phase I Studies (Chi et al, Annals Oncol 27(6):1116-1122, 2016)
Dose-dependent grade 1-2 infusion reactions
Reductions in tumor markers in patients with prostate and ovarian cancer
Declines in circulating tumour cells (CTC) observed¥
Randomized Phase II study in Treatment Naïve CRPC (Chi et al, J Clin Oncol 30, 2012 (suppl; abstr 4514)
PSA ³50% decline in 41% (OGX-427) vs 20% (Prednisone)
Figure 1. Apatorsen suppresses Pb-Luc Bioluminescence, AR, Hsp90 and Hsp27 in vivo (LNCaP)
Figure 2. Apatorsen prevents metastases (PC-3M)
ASO Scr
427 OGX -
Zoubeidi et al, Cancer Res, 67:10455, 2007
OBJECTIVES
Primary
Progression-Free Survival at the milestone Day 60 assessment: to ascertain whether Apatorsen added to Abiraterone-Prednisone has a greater proportion of patients observed to be alive without progression at Day 60 (±7 days) as compared to continuing Abiraterone- Prednisone in men with mCRPC and PSA progression on Abiraterone-Prednisone.
Disease progression is defined by any one of the following: Measurable disease: progression per RECIST 1.1 by CT; Bone scan: ³ 2 new lesions confirmed; Disease related deterioration of health status; Need for palliative radiation therapy
Secondary
The proportion of patients who have a PSA response (³ 30% decline) and any PSA decline post-randomization
Objective response rate
Progression-free survival (PFS)
Time to disease progression
Circulating tumor cell (CTC) counts at baseline and on study
STUDY DESIGN
Metastatic CRPC
PSA Progression While Receiving Abiraterone-Prednisone
1:1 Stratified by
Prior chemotherapy (Yes vs No)
Prior PSA decline ³30% (Yes vs No)
[Graphic Appears Here]
[Graphic Appears Here]
[Graphic Appears Here]
ARM A ARM B
Abiraterone: 1000 mg PO OD Abiraterone: 1000 mg PO OD
Prednisone: 5 mg PO BID Prednisone: 5 mg PO BID
Apatorsen: 600 mg IV x 3 loading doses
then 800 mg IV weekly x 48 weeks
Cross-over at progression
Key Inclusion Criteria
Metastatic CRPC
ECOG performance status 0-1
Evidence of PSA progression only while on Abiraterone and Prednisone
0-1 prior lines of chemotherapy for CRPC
Baseline laboratory values:
ANC ³ 1.5 x 109 cells /L, platelet count ³ 100 x 109 /L, and hemoglobin ³ 9 g/dL
Creatinine £ 1.3 x ULN
Total bilirubin £ 1.1 x ULN, ALT and AST £ 3.0 x ULN
Statistical Considerations
Planned sample size was 74 patients with patients randomized with equal probability to the two treatment arms.
The one-sided type I error probability for the outcome is specified to be 10%, giving an overall type I error probability for the primary endpoint of 20%. Assuming control arm success probability is 5%, then there will be 90% power to detect a hypothesized 25% increment in the success probability
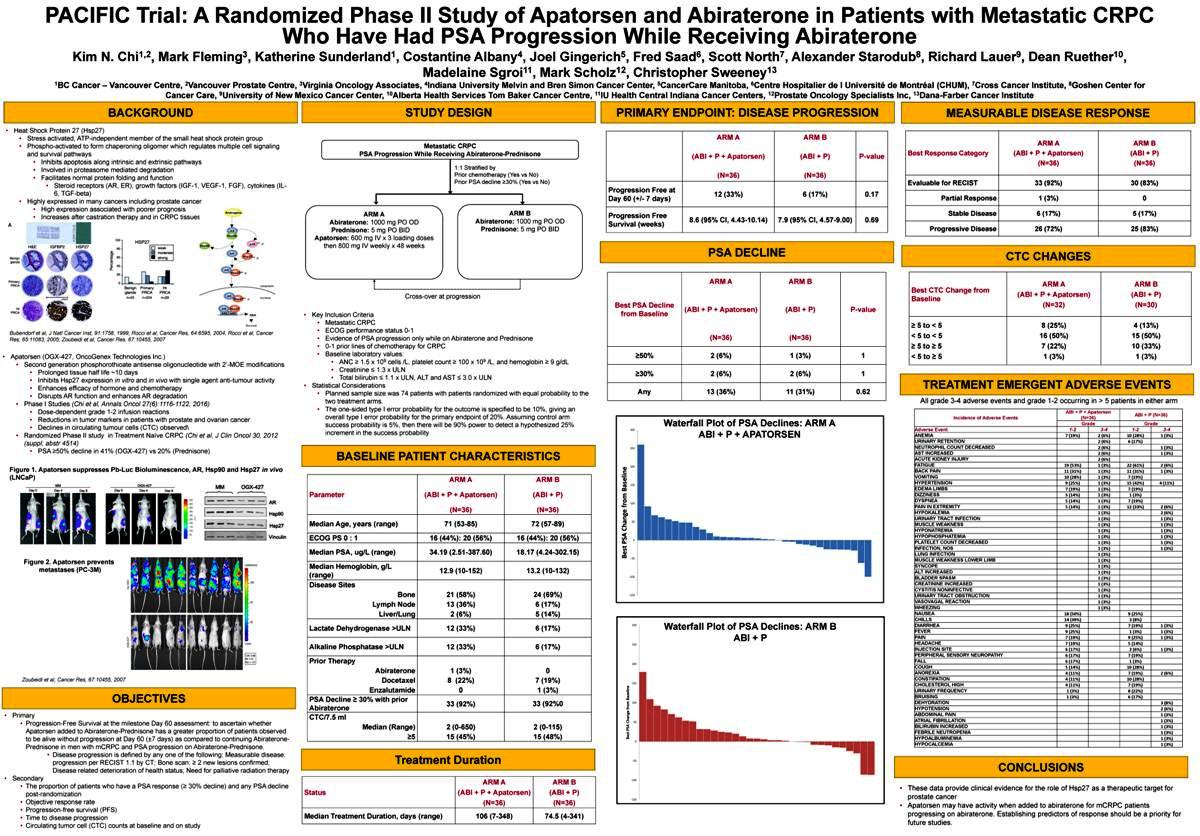
BASELINE PATIENT CHARACTERISTICS
ARM A ARM B
Parameter (ABI + P + Apatorsen) (ABI + P)
(N=36) (N=36)
Median Age, years (range) 71 (53-85) 72 (57-89)
ECOG PS 0 : 1 16 (44%): 20 (56%) 16 (44%): 20 (56%)
Median PSA, ug/L (range) 34.19 (2.51-387.60) 18.17 (4.24-302.15)
Median Hemoglobin, g/L
(range) 12.9 (10-152)13.2 (10-132)
Disease Sites
Bone 21 (58%)24 (69%)
Lymph Node 13 (36%)6 (17%)
Liver/Lung 2 (6%)5 (14%)
Lactate Dehydrogenase >ULN 12 (33%)6 (17%)
Alkaline Phosphatase >ULN 12 (33%)6 (17%)
Prior Therapy
Abiraterone 1 (3%)0
Docetaxel 8 (22%)7 (19%)
Enzalutamide 01 (3%)
PSA Decline ³ 30% with prior
Abiraterone 33 (92%)33 (92%0
CTC/7.5 ml
Median (Range) 2 (0-650) 2 (0-115)
³5 15 (45%)15 (48%)
Treatment Duration
ARM AARM B
Status (ABI+ P + Apatorsen)(ABI + P)
(N=36)(N=36)
Median Treatment Duration,days(range)106 (7-348)74.5 (4-341)
PRIMARY ENDPOINT: DISEASE PROGRESSION
ARM A ARM B
(ABI + P + Apatorsen) (ABI + P)P-value
(N=36) (N=36)
Progression Free at
Day 60 (+/- 7 days) 12 (33%) 6 (17%)0.17
Progression Free 8.6 (95% CI, 4.43-10.14) 7.9(95% CI, 4.57-9.00)0.69
Survival (weeks)
PSA DECLINE
ARM A ARM B
Best PSA Decline
(ABI + P + Apatorsen) (ABI + P) P-value from Baseline
(N=36) (N=36)
³50% 2 (6%) 1 (3%) 1 ³30% 2 (6%) 2 (6%) 1 Any 13 (36%) 11 (31%) 0.62
Waterfall Plot of PSA Declines: ARM A 300 ABI + P + APATORSEN
250
200
Baseline 150 from 100 Change 50 PSA 0 Best
-50 -100 -150
300 Waterfall Plot of PSA Declines: ARM B ABI + P
250 200
150
Baseline from 100 Change 50 PSA Best
0 -50 -100 -150
MEASURABLE DISEASE RESPONSE
ARM AARM B
Best Response Category (ABI +P + Apatorsen)(ABI + P)
(N=36)(N=36)
Evaluable for RECIST 33(92%)30 (83%)
Partial Response 1(3%)0
Stable Disease 6(17%)5 (17%)
Progressive Disease 26(72%)25 (83%)
CTC CHANGES
ARM AARM B
Best CTC Change from (ABI +P + Apatorsen)(ABI + P)
Baseline
(N=32)(N=30)
³ 5 to < 5 8 (25%)4(13%)
< 5 to < 5 16 (50%)15(50%)
³ 5 to ³ 5 7 (22%)10(33%)
< 5 to ³ 5 1 (3%)1(3%)
TREATMENT EMERGENT ADVERSE EVENTS
All grade 3-4 adverse events and grade 1-2 occurring in > 5 patients in either arm
ABI + P + Apatorsen ABI + P (N=36)
Incidence of Adverse Events (N=36)
GradeGrade
Adverse Event 1-23-41-23-4
ANEMIA 7 (19%)2(6%)10 (28%)1(3%)
URINARY RETENTION 2(6%)6(17%)
NEUTROPHIL COUNT DECREASED 2(6%)1(3%)
AST INCREASED 2(6%)1(3%)
ACUTE KIDNEY INJURY 2(6%)
FATIGUE 19 (53%) 1(3%)22 (61%)2(6%)
BACK PAIN 11 (31%) 1(3%)11 (31%)1(3%)
VOMITING 10 (28%) 1(3%)7(19%)
HYPERTENSION 9 (25%)1(3%)15 (42%)4 (11%)
EDEMA LIMBS 7 (19%)1(3%)7(19%)
DIZZINESS 5 (14%)1(3%)1 (3%)
DYSPNEA 5 (14%)1(3%)7(19%)
PAIN IN EXTREMITY 5 (14%)1(3%)12 (33%)2(6%)
HYPOKALEMIA 1(3%)2(6%)
URINARY TRACT INFECTION 1(3%)1(3%)
MUSCLE WEAKNESS 1(3%)1(3%)
HYPONATREMIA 1(3%)1(3%)
HYPOPHOSPHATEMIA 1(3%)1(3%)
PLATELET COUNT DECREASED 1(3%)1(3%)
INFECTION, NOS 1(3%)1(3%)
LUNG INFECTION 1(3%)
MUSCLE WEAKNESS LOWER LIMB 1(3%)
SYNCOPE 1(3%)
ALT INCREASED 1(3%)
BLADDER SPASM 1(3%)
CREATININE INCREASED 1(3%)
CYSTITIS NONINFECTIVE 1(3%)
URINARY TRACT OBSTRUCTION 1(3%)
VASOVAGAL REACTION 1(3%)
WHEEZING 1(3%)
NAUSEA 18 (50%) 9(25%)
CHILLS 14 (39%) 3 (8%)
DIARRHEA 9 (25%)7(19%)1(3%)
FEVER 9 (25%)1 (3%)1(3%)
PAIN 7 (19%)9(25%)1(3%)
HEADACHE 7 (19%)5(14%)
INJECTION SITE 6 (17%)2 (6%)1(3%)
PERIPHERAL SENSORY NEUROPATHY 6 (17%)7(19%)
FALL 6 (17%)1 (3%)
COUGH 5 (14%)10 (28%)
ANOREXIA 4 (11%)7(19%)2(6%)
CONSTIPATION 4 (11%)10 (28%)
CHOLESTEROL HIGH 4 (11%)7(19%)
URINARY FREQUENCY 1 (3%) 8(22%)
BRUISING 1 (3%) 6(17%)
DEHYDRATION 3(8%)
HYPOTENSION 2(6%)
ABDOMINAL PAIN 1(3%)
ATRIAL FIBRILLATION 1(3%)
BILIRUBIN INCREASED 1(3%)
FEBRILE NEUTROPENIA 1(3%)
HYPOALBUMINEMIA 1 (3%)
HYPOCALCEMIA 1 (3%)
CONCLUSIONS
These data provide clinical evidence for the role of Hsp27 as a therapeutic target for prostate cancer
Apatorsen may have activity when added to abiraterone for mCRPC patients progressing on abiraterone. Establishing predictors of response should be a priority for future studies.
|
|
Borealis-2: A randomized phase II study of OGX-427 (apatorsen) plus docetaxel versus docetaxel alone in platinum-resistant metastatic urothelial cancer (mUC)
TK Choueiri1, N. Hahn2, M. Regan1, L. Werner1, A. Alva3 , S. George4, J. Picus5, R. Alter6, A. Balar7, J. Hoffman-Censits8, P. Grivas9, R. Lauer10, E. Guancial11, C. Hoimes12, G. Sonpavde13 , C. Albany14, M. Stein15, C. Jacobs16, P. Stewart16, A. Lalani1, S. Pal17, JE Rosenberg18
[Graphic Appears Here]
1Dana-Farber Cancer Institute, Boston MA,2Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore MD, 3University of Michigan Comprehensive Cancer Center, Ann Arbor MI, 4Roswell Park Cancer Institute, Buffalo NY, 5Siteman Cancer Center, Washington University, St. Louis MO, 6John Theurer Cancer Center, Hackensack University
Medical Center, Hackensack NJ, 7New YorkUniversity Perlmutter Cancer Center, New York NY, 8Thomas Jefferson University Kimmel Cancer Center, Philadelphia PA, 9Cleveland Clinic Taussig Cancer Institute, Cleveland OH, 10University of New Mexico Cancer Center, Albuquerque NM, 11University of Rochester Wilmot Cancer Center, Rochester NY, 12University
ABSTRACT #289 Hospitals Cleveland Medical Center, Cleveland OH, 13University of Alabama Comprehensive Cancer Center, Birmingham AL, 14Indiana University Melvin and Bren Simon Cancer Center, Indiana IN, 15Rutgers Cancer Institute of New Jersey, New Brunswick NJ, 16OncoGenex Pharmaceuticals, Inc., Bothell WA, 17City of Hope Comprehensive Cancer Center, Duarte CA,NCT01780545
18Memorial Sloan Kettering Cancer Center, New York NY
INTRODUCTION
Heat shock protein 27 (Hsp27) is over-expressed in many malignancies including bladder cancer. Increased Hsp27 has been associated with inhibition of chemotherapy-induced apoptosis, increased tumor cytoprotection, and development of treatment resistance.
Apatorsen (OGX-427), a 2nd generation antisense oligonucleotide that inhibits expression of Hsp27, has been shown to inhibit tumor growth and sensitize tumor cells to chemotherapy in a variety of malignancies, including urothelial cancer.
Results of preclinical and phase 1 studies suggest that addition of apatorsen to chemotherapy is well tolerated and may improve treatment efficacy.
Borealis-2 is a randomized, multicenter, phase 2 study of apatorsen in combination with docetaxel (DOC) vs. DOC alone in locally advanced/metastatic bladder cancer patients who received at least one line of prior platinum-based therapy.
OBJECTIVES
PRIMARY:
Determine whether DOC administered in combination with apatorsen improves overall survival (OS) compared to DOC alone
SECONDARY:
Safety and tolerability
Compare overall response rate (ORR) and progression-free survival (PFS) rates between the arms
Evaluate the effect of each arm on serum Hsp27 levels
Evaluate the association of urothelial carcinoma expression of Hsp27 measured by IHC in archival tissue
Evaluate the effect of therapy on peripheral blood circulating tumor cells (CTCs)
PATIENTS and METHODS
SCREEN AND STRATIFY ELGIBLE PATIENTS* BETWEEN 8/2013 - 9/2015: *Eligibility:
Time from prior systemic therapy (< 3 vs. ³ 3 months) Age > 18 years
Bellmunt criteria (0 versus 1-3 risk factors)1 ECOG Performance Status 0 or 1
Histologically confirmed metastatic
or inoperable, locally-advanced
RANDOMIZATION 1:1 urothelial carcinoma
N=200Measurable disease by RECIST v1.1
criteria
Received prior systemic platinum-
based chemotherapy for urothelial
Experimental Arm (A) n=99: Control Arm (B) n=101: carcinoma
Apatorsen: Loading doses Docetaxel**: Starting within 5
(3 x at 600 mg IV days -9 to -1), days of randomization,
followed by weekly doses 75 mg/m2 IV
+ every 21 days
Docetaxel**: 75 mg/m2 IV
every 21 days (Maximum 10 docetaxel cycles)** Docetaxel Exposure:
(Maximum 10 docetaxel cycles) Both arms received a median of 2
cycles docetaxel (range 1-10) during
then study treatment
Maintenance Apatorsen:
in patients who do not have disease progression or
who discontinue docetaxel due to toxicity without
disease progression and have completed disease
assessments following at least 2 cycles of
chemotherapy. Maintenance treatment until
documented disease progression or unacceptable
toxicity
[Graphic Appears Here]
FOLLOW FOR SURVIVAL
Primary end point: OS
Secondary end points: Safety, ORR, PFS, CTCs
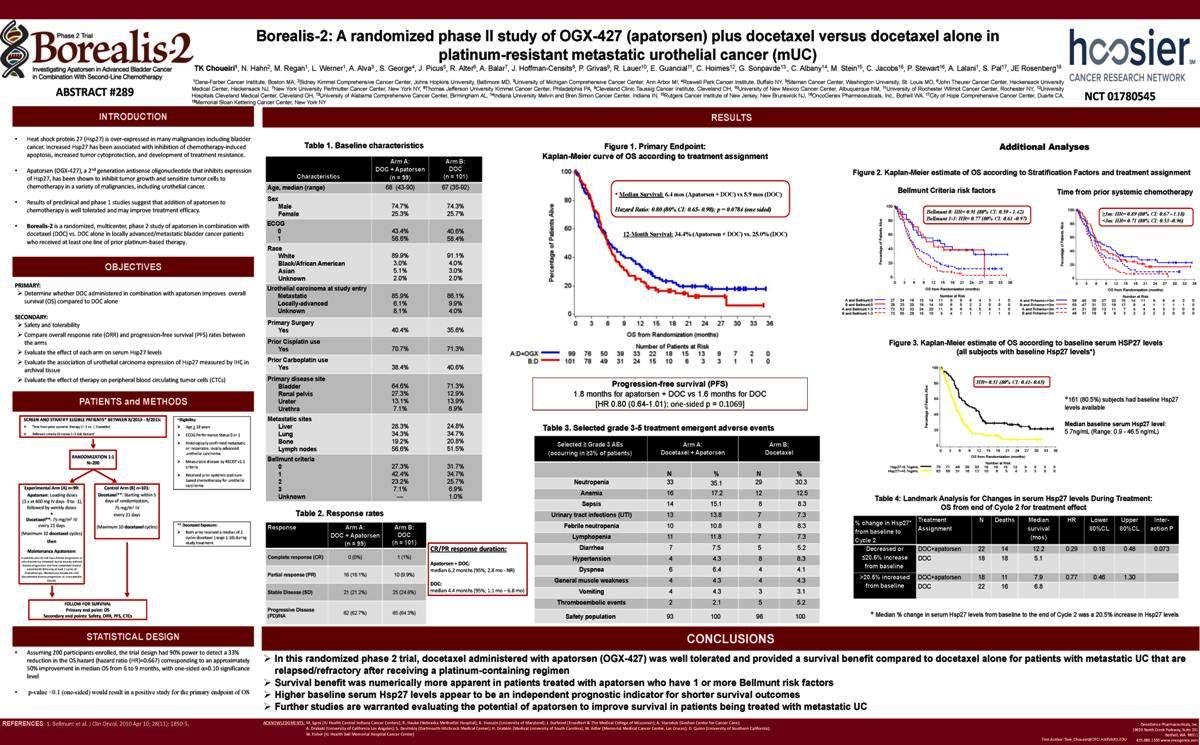
Table 1. Baseline characteristics
Arm A:Arm B:
DOC + Apatorsen DOC
Characteristics (n = 99) (n = 101)
Age, median (range) 68 (43-90)67 (35-92)
Sex
Male 74.7%74.3%
Female 25.3%25.7%
ECOG
0 43.4%40.6%
1 56.6%58.4%
Race
White 89.9%91.1%
Black/African American 3.0% 4.0%
Asian 5.1%3.0%
Unknown 2.0%2.0%
Urothelial carcinoma at study entry
Metastatic 85.9%86.1%
Locally-advanced 6.1%9.9%
Unknown 8.1%4.0%
Primary Surgery
Yes 40.4%35.6%
Prior Cisplatin use
Yes 70.7%71.3%
Prior Carboplatin use
Yes 38.4%40.6%
Primary disease site
Bladder 64.6%71.3%
Renal pelvis 27.3%12.9%
Ureter 13.1%13.9%
Urethra 7.1%8.9%
Metastatic sites
Liver 28.3%24.8%
Lung 34.3%34.7%
Bone 19.2%20.8%
Lymph nodes 56.6%51.5%
Bellmunt criteria
0 27.3%31.7%
1 42.4%34.7%
2 23.2%25.7%
3 7.1%6.9%
Unknown ---1.0%
Table 2. Response rates
Response Arm A: Arm B:
DOC + Apatorsen DOC
(n = 99) (n = 101)CR/PR response duration:
Complete response (CR) 0 (0%) 1 (1%)
Apatorsen + DOC:
median 6.2 months (95%; 2.8 mo - NR)
Partial response (PR) 16 (16.1%) 10 (9.9%)
DOC:
Stable Disease (SD) 21 (21.2%) 25 (24.8%)median 4.4 months (95%; 1.1 mo 6.8 mo)
Progressive Disease
(PD)/NA 62 (62.7%) 65 (64.3%)
[Graphic Appears Here]
Progression-free survival (PFS)
1.8 months for apatorsen + DOC vs 1.6 months for DOC [HR 0.80 (0.64-1.01); one-sided p = 0.1069]
Table 3. Selected grade 3-5 treatment emergent adverse events
Selected ³ Grade 3 AEs Arm A:Arm B:
(occurring in ³3% of patients) Docetaxel + Apatorsen Docetaxel
N %N%
Neutropenia 33 35.12930.3
Anemia 16 17.21212.5
Sepsis 14 15.188.3
Urinary tract infections (UTI) 13 13.877.3
Febrile neutropenia 10 10.888.3
Lymphopenia 11 11.877.3
Diarrhea 7 7.555.2
Hypertension 4 4.388.3
Dyspnea 6 6.444.1
General muscle weakness 4 4.344.3
Vomiting 4 4.333.1
Thromboembolic events 2 2.155.2
Safety population 93 10096100
[Graphic Appears Here]
Table 4: Landmark Analysis for Changes in serum Hsp27 levels During Treatment: OS from end of Cycle 2 for treatment effect
% change in Hsp27* Treatment NDeathsMedianHRLowerUpperInter-
from baseline to Assignment survival80%CL80%CLaction P
Cycle 2 (mos)
Decreased or DOC+apatorsen 221412.20.290.180.480.073
£20.5% increase DOC 18185.1
from baseline
>20.5% increased DOC+apatorsen 18117.90.770.461.30
from baseline DOC 22166.8
* Median % change in serum Hsp27 levels from baseline to the end of Cycle 2 was a 20.5% increase in Hsp27 levels
STATISTICAL DESIGN
Assuming 200 participants enrolled, the trial design had 90% power to detect a 33% reduction in the OS hazard (hazard ratio (HR)=0.667) corresponding to an approximately 50% improvement in median OS from 6 to 9 months, with one-sided á=0.10 significance level
p-value <0.1 (one-sided) would result in a positive study for the primary endpoint of OS
REFERENCES: 1. Bellmunt et al. J Clin Oncol. 2010 Apr 10; 28(11): 1850-5.
CONCLUSIONS
In this randomized phase 2 trial, docetaxel administered with apatorsen (OGX-427) was well tolerated and provided a survival benefit compared to docetaxel alone for patients with metastatic UC that are relapsed/refractory after receiving a platinum-containing regimen
Survival benefit was numerically more apparent in patients treated with apatorsen who have 1 or more Bellmunt risk factors
Higher baseline serum Hsp27 levels appear to be an independent prognostic indicator for shorter survival outcomes
Further studies are warranted evaluating the potential of apatorsen to improve survival in patients being treated with metastatic UC
ACKNOWLEDGMENTS: M. Sgroi (IU Health Central Indiana Cancer Centers); R. Hauke (Nebraska Methodist Hospital); A. Hussain (University of Maryland); J. Burfeind (Froedtert & The Medical College of Wisconsin); A. Starodub (Goshen Center for Cancer Care); OncoGenex Pharmaceuticals, Inc.
A. Drakaki (University of California Los Angeles); S. Devitskiy (Dartmouth-Hitchcock Medical Center); H. Drabkin (Medical University of South Carolina); W. Adler (Memorial Medical Cancer Center, Las Cruces); D. Quinn (University of Southern California); 19820 North Creek Parkway, Suite 201
W. Fisher (IU Health Ball Memorial Hospital Cancer Center) Bothell, WA 98011
First Author: Toni_Choueiri@DFCI.HARVARD.EDU425.686.1500 www.oncogenex.com