DEFA14A: Additional definitive proxy soliciting materials and Rule 14(a)(12) material
Published on July 21, 2008
|
UNITED STATES |
|||
|
SECURITIES AND EXCHANGE COMMISSION |
|||
|
Washington, D.C. 20549 |
|||
|
|
|||
|
SCHEDULE 14A |
|||
|
|
|||
|
Proxy Statement
Pursuant to Section 14(a) of |
|||
|
|
|||
|
Filed by the Registrant x |
|||
|
|
|||
|
Filed by a Party other than the Registrant o |
|||
|
|
|||
|
Check the appropriate box: |
|||
|
o |
Preliminary Proxy Statement |
||
|
o |
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
||
|
o |
Definitive Proxy Statement |
||
|
x |
Definitive Additional Materials |
||
|
o |
Soliciting Material Pursuant to §240.14a-12 |
||
|
|
|||
|
Sonus Pharmaceuticals, Inc. |
|||
|
(Name of Registrant as Specified In Its Charter) |
|||
|
|
|||
|
|
|||
|
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
|||
|
|
|||
|
Payment of Filing Fee (Check the appropriate box): |
|||
|
x |
No fee required. |
||
|
o |
Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
||
|
|
(1) |
Title of each class of securities to which transaction applies: |
|
|
|
|
|
|
|
|
(2) |
Aggregate number of securities to which transaction applies: |
|
|
|
|
|
|
|
|
(3) |
Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
|
|
|
|
|
|
|
|
(4) |
Proposed maximum aggregate value of transaction: |
|
|
|
|
|
|
|
|
(5) |
Total fee paid: |
|
|
|
|
|
|
|
o |
Fee paid previously with preliminary materials. |
||
|
o |
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
||
|
|
(1) |
Amount Previously Paid: |
|
|
|
|
|
|
|
|
(2) |
Form, Schedule or Registration Statement No.: |
|
|
|
|
|
|
|
|
(3) |
Filing Party: |
|
|
|
|
|
|
|
|
(4) |
Date Filed: |
|
|
|
|
|
|
|
|
OncoGenex Pharmaceuticals, Inc. Merger of Sonus Pharmaceuticals Inc. and OncoGenex Technologies Inc. Committed to the development of new cancer therapies that address unmet needs in the treatment of cancer July 17, 2008 Bringing hope to life. |
|
|
2 Confidential Forward-Looking Statements This presentation contains forward-looking statements based upon current expectations and beliefs that involve a number of significant risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. All statements other than historical facts are statements that could be deemed forward-looking. Potential risks and uncertainties, include, among others, the possibility that the merger does not close or that the closing may be delayed; synergies and costs savings may not be achieved and we may be unable to successfully execute our integration strategies; the timing and costs of clinical trials and regulatory approvals are uncertain; our clinical trials may not be successful; preclinical studies may not be indicative of clinical trial results; risks associated with obtaining funding from third parties or completing a financing necessary to support the costs and expenses of clinical studies as well as research and development activities; risks associated with our reliance on intellectual property licenses from third parties; our potential inability to protect and enforce our intellectual property rights; risks that we will not be able to maintain listing on NASDAQ, as well as other risks relating to the development, safety and efficacy of therapeutic drugs and potential applications for these products. No assurance can be given that any of the events anticipated by the forward-looking statements will occur, or if any of them do, what impact they will have on our results of operations or financial condition. We undertake no obligation to update the forward-looking statements contained herein or to reflect events or circumstances occurring after the date hereof. |
|
|
3 Confidential OncoGenex Pharmaceuticals, Inc.: An Overview of the Combined Company OncoGenex and Sonus (NASDAQ: SNUS) announced signing of definitive agreement to merger; planned closing August 20, 2008 Focused on the development of novel therapeutics for patients with cancer Four distinct product candidates in development Lead product candidate in 5 Phase 2 clinical trials Preliminary data support advancing development in HRPC and NSCLC Initial focus will be in hormone refractory prostate cancer Two product candidates in Phase 1 with plans to transition to Phase 2 One pre-clinical product candidate with future plans to transition to Phase 1 Near term development milestones Experienced drug development team with multiple product approvals |
|
|
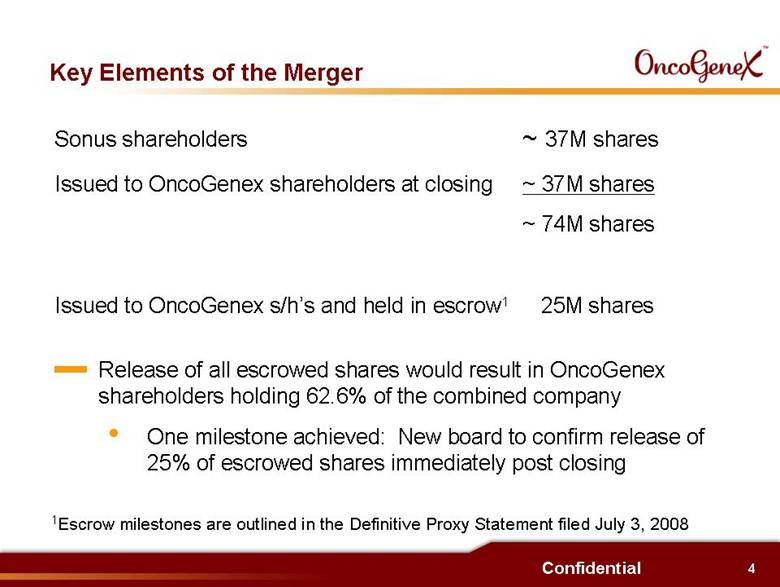
4 Confidential Key Elements of the Merger Sonus shareholders ~ 37M shares Issued to OncoGenex shareholders at closing ~ 37M shares ~ 74M shares Issued to OncoGenex s/hs and held in escrow1 25M shares Release of all escrowed shares would result in OncoGenex shareholders holding 62.6% of the combined company One milestone achieved: New board to confirm release of 25% of escrowed shares immediately post closing 1Escrow milestones are outlined in the Definitive Proxy Statement filed July 3, 2008 |
|
|
5 Confidential Key Elements of the Merger (Continued) Post-merger Board of Directors will be comprised of six members equally designated from the current boards of Sonus and OncoGenex A 7th independent director will be appointed by new Board of Directors The Chief Executive Officer and Chief Financial Officer of OncoGenex will assume those roles for the combined entity Sonus will change its name to OncoGenex Pharmaceuticals, Inc. and its ticker symbol to OGXI. |
|
|
6 Confidential Management & the Board Post Merger Management Team1 Scott D. Cormack President & CEO (from OncoGenex) Steve Anderson, MBA, CA Chief Financial Officer (from OncoGenex) Cindy Jacobs, M.D., Ph.D. Chief Medical Officer (from OncoGenex) Monica Krieger, Ph.D. VP, Regulatory Affairs (from OncoGenex) Elaine Waller, Pharm. D. SVP, Regulatory/Quality and Clinical Research (from Sonus) Martin E. Gleave, M.D. Chief Scientific Officer (from OncoGenex) Dean Kessler, DABT VP, Preclinical Development (from Sonus) Board of Directors Scott D. Cormack President and CEO, OncoGenex Michelle Burris CFO, Trubion Pharmaceuticals Neil Clendeninn, M.D., Ph.D. Former Exec VP, Head of Clinical Dev Agouron Pharmaceuticals Dwight Winstead Group President, Clinical Technologies and Services, Cardinal Health Michael A. Martino Former CEO, Sonus Pharmaceuticals Patrick Brady Vice President, GrowthWorks 7th Member to Be Appointed by Board 1 Position titles are pre-merger titles; some will change post-merger |
|
|
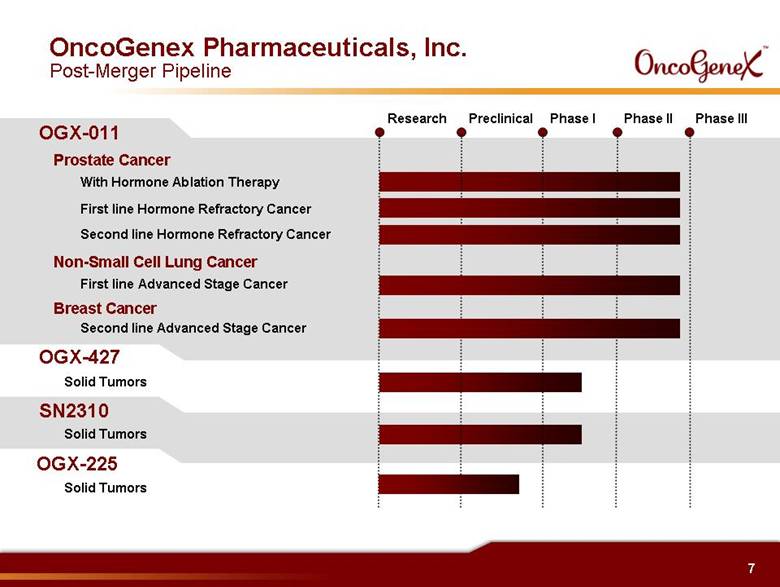
7 With Hormone Ablation Therapy First line Hormone Refractory Cancer OGX-011 Prostate Cancer Second line Hormone Refractory Cancer First line Advanced Stage Cancer Non-Small Cell Lung Cancer Breast Cancer Second line Advanced Stage Cancer Phase I Preclinical Research Phase II Phase III OGX-427 Solid Tumors OncoGenex Pharmaceuticals, Inc. Post-Merger Pipeline SN2310 Solid Tumors OGX-225 Solid Tumors |
|
|
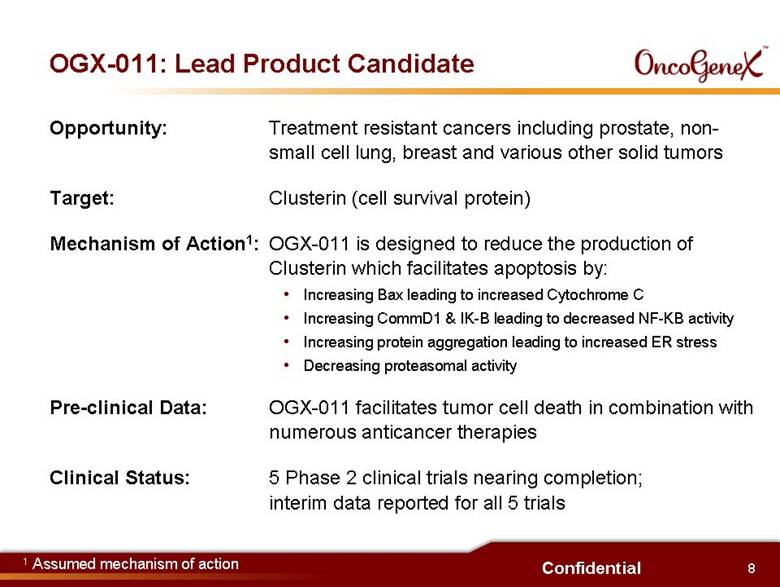
8 Confidential OGX-011: Lead Product Candidate Opportunity: Treatment resistant cancers including prostate, non-small cell lung, breast and various other solid tumors Target: Clusterin (cell survival protein) Mechanism of Action1: OGX-011 is designed to reduce the production of Clusterin which facilitates apoptosis by: Increasing Bax leading to increased Cytochrome C Increasing CommD1 & IK-B leading to decreased NF-KB activity Increasing protein aggregation leading to increased ER stress Decreasing proteasomal activity Pre-clinical Data: OGX-011 facilitates tumor cell death in combination with numerous anticancer therapies Clinical Status: 5 Phase 2 clinical trials nearing completion; interim data reported for all 5 trials 1 Assumed mechanism of action |
|
|
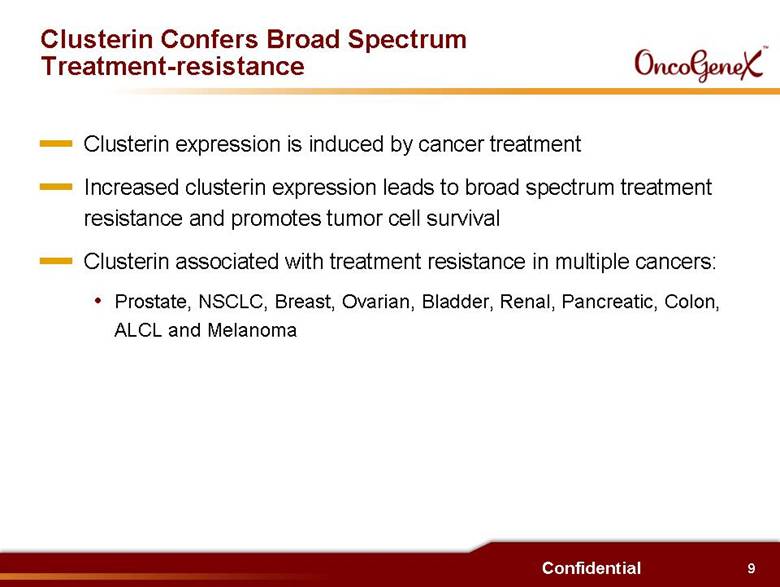
9 Confidential Clusterin Confers Broad Spectrum Treatment-resistance Clusterin expression is induced by cancer treatment . Increased clusterin expression leads to broad spectrum treatment resistance and promotes tumor cell survival Clusterin associated with treatment resistance in multiple cancers: Prostate, NSCLC, Breast, Ovarian, Bladder, Renal, Pancreatic, Colon, ALCL and Melanoma |
|
|
10 Confidential OGX-011 Clinical Program Chart 81 patients; Fully enrolled 1st line chemotherapy (docetaxel) with and without OGX-011 HRPC 67 patients; Fully enrolled OGX-011 with 2nd line chemotherapy (docetaxel or mitoxantrone) HRPC 15 patients; Fully enrolled OGX-011 with 2nd line chemotherapy (docetaxel) Advanced Breast Cancer 23 patients; Fully enrolled OGX-011 with hormone ablation therapy Localized Prostate Cancer 81 patients; Fully enrolled OGX-011 with 1st line chemotherapy (gemcitabine/cisplatin or carboplatin) Advanced NSCLC Status Treatment Indication Over 300 patients enrolled in OGX-011 clinical trials Phase 1 Clinical Trials - Completed : Phase 2 Clinical Trials Follow Up Ongoing: 40 patients; Trial completed OGX-011 with chemotherapy (docetaxel) Solid Tumors 25 patients; Trial completed OGX-011 with hormone ablation therapy Localized Prostate Cancer Status Treatment Indication |
|
|
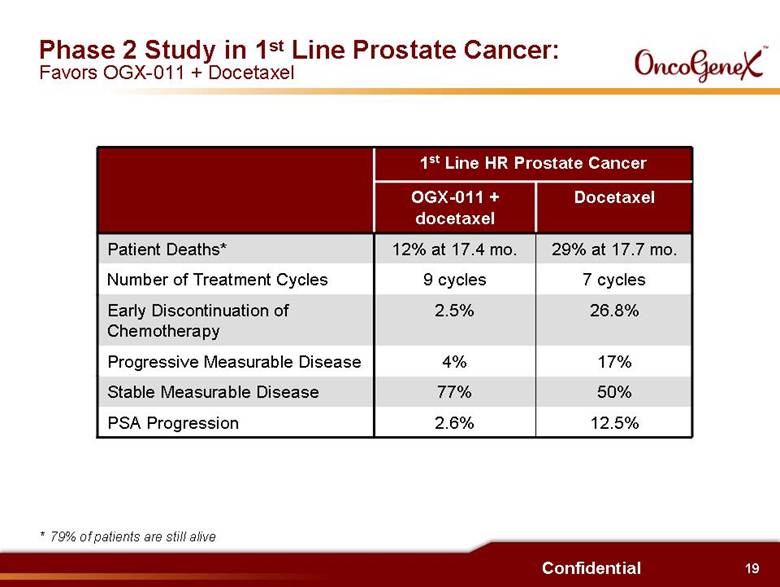
11 Confidential Potential Benefits of OGX-011 in Oncology Interim results from Phase 2 trials: . Increased survival duration when OGX-011 added to chemotherapy: 14.7 months vs. ~ 10 months in 2nd Line HRPC (docetaxel) 11.4 months vs. ~ 10 months in 2nd Line HRPC (mitoxantrone) 14.1 months vs. 8 10.8 months in 1st Line NSCLC . Greater pain control (even when compared to 1st Line results): 61% pain reduction in 2nd Line HRPC vs. 35% in 1st Line HRPC (docetaxel) 46% pain reduction in 2nd Line HRPC vs. 22% in 1st Line HRPC (mitoxantrone) . Decreased treatment failure: 4% vs. 17% progressive measurable disease in 1st Line HRPC randomized trial 2.6% vs. 12.5% PSA progression in 1st Line HRPC randomized trial |
|
|
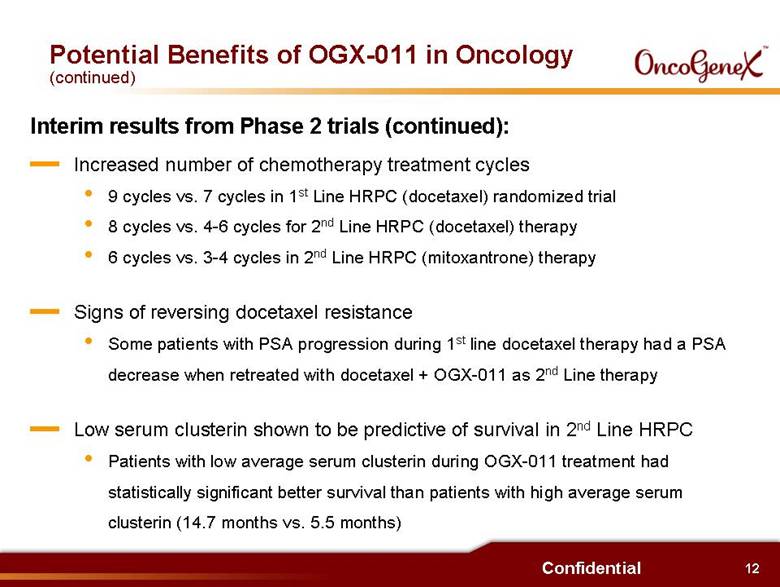
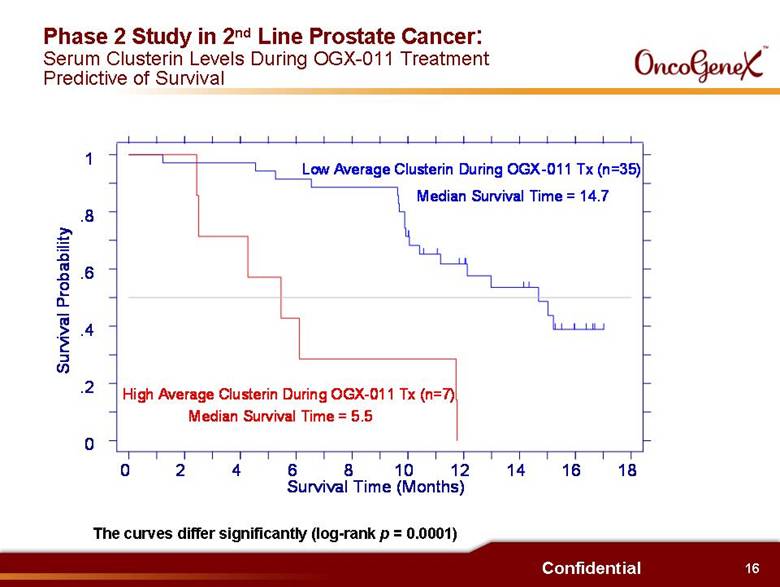
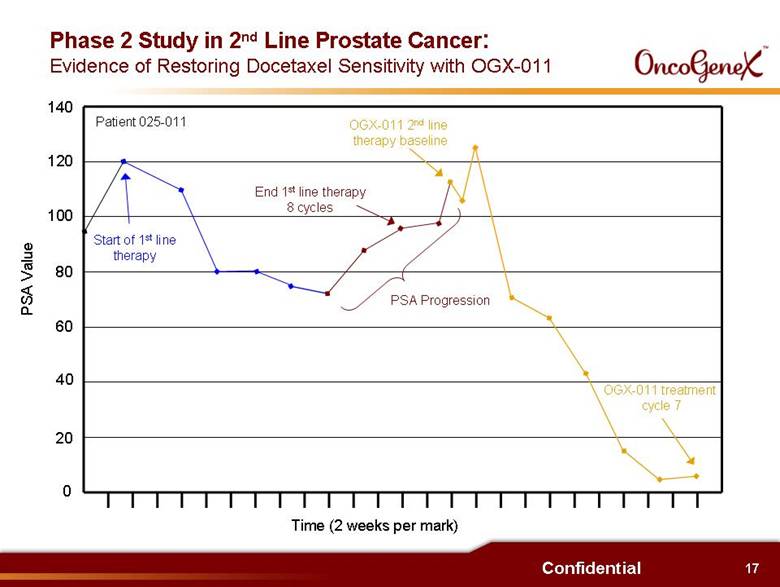
12 Confidential Potential Benefits of OGX-011 in Oncology (continued) Interim results from Phase 2 trials (continued): . Increased number of chemotherapy treatment cycles 9 cycles vs. 7 cycles in 1st Line HRPC (docetaxel) randomized trial 8 cycles vs. 4-6 cycles for 2nd Line HRPC (docetaxel) therapy 6 cycles vs. 3-4 cycles in 2nd Line HRPC (mitoxantrone) therapy . Signs of reversing docetaxel resistance Some patients with PSA progression during 1st line docetaxel therapy had a PSA decrease when retreated with docetaxel + OGX-011 as 2nd Line therapy . Low serum clusterin shown to be predictive of survival in 2nd Line HRPC Patients with low average serum clusterin during OGX-011 treatment had statistically significant better survival than patients with high average serum clusterin (14.7 months vs. 5.5 months) |
|
|
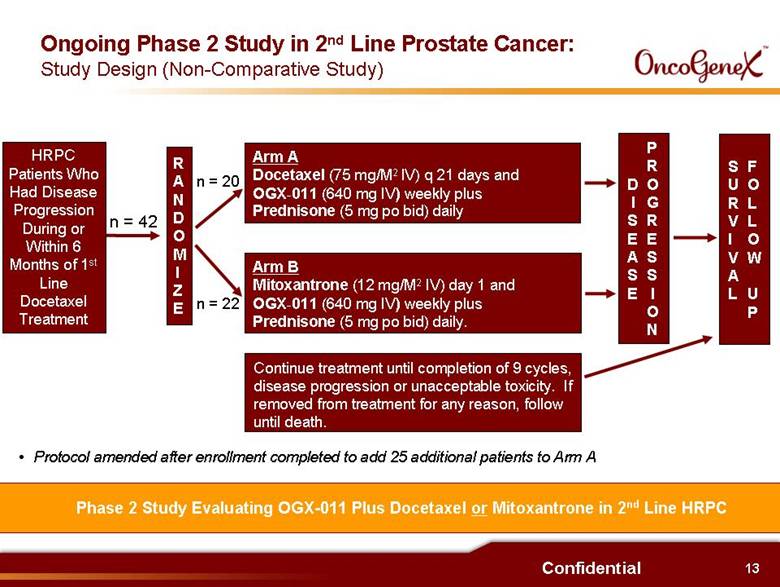
13 Confidential Phase 2 Study Evaluating OGX-011 Plus Docetaxel or Mitoxantrone in 2nd Line HRPC Ongoing Phase 2 Study in 2nd Line Prostate Cancer: Study Design (Non-Comparative Study) RANDOMIZE Arm A Docetaxel (75 mg/M2 IV) q 21 days and OGX-011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily Arm B Mitoxantrone (12 mg/M2 IV) day 1 and OGX-011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily. Continue treatment until completion of 9 cycles, disease progression or unacceptable toxicity. If removed from treatment for any reason, follow until death. DISEASE PROGRESSION SURVIVAL FOLLOW UP n = 42 n = 20 n = 22 Protocol amended after enrollment completed to add 25 additional patients to Arm A HRPC Patients Who Had Disease Progression During or Within 6 Months of 1st Line Docetaxel Treatment |
|
|
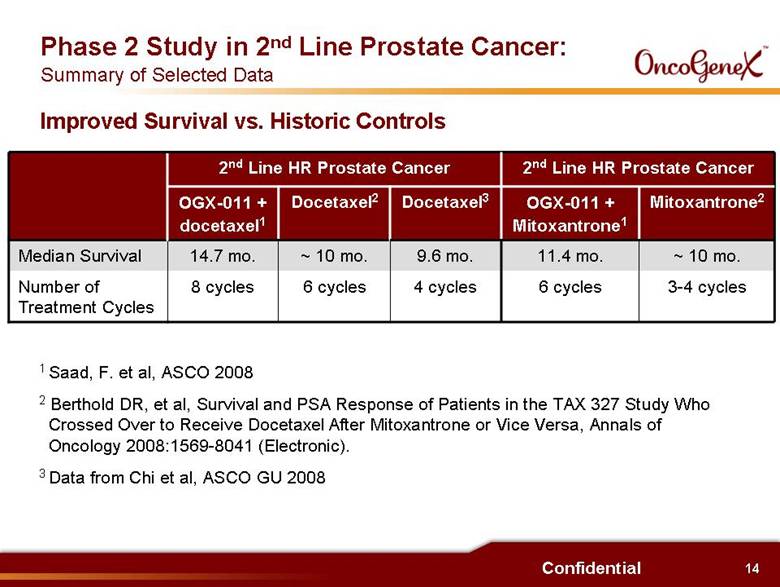
14 Confidential Phase 2 Study in 2nd Line Prostate Cancer: Summary of Selected Data 6 cycles ~ 10 mo. Docetaxel2 4 cycles 9.6 mo. Docetaxel3 8 cycles 14.7 mo. OGX-011 + docetaxel1 2nd Line HR Prostate Cancer Mitoxantrone2 OGX-011 + Mitoxantrone1 6 cycles 11.4 mo. 2nd Line HR Prostate Cancer 3-4 cycles ~ 10 mo. Number of Treatment Cycles Median Survival 1 Saad, F. et al, ASCO 2008 2 Berthold DR, et al, Survival and PSA Response of Patients in the TAX 327 Study Who Crossed Over to Receive Docetaxel After Mitoxantrone or Vice Versa, Annals of Oncology 2008:1569-8041 (Electronic). 3 Data from Chi et al, ASCO GU 2008 Improved Survival vs. Historic Controls |
|
|
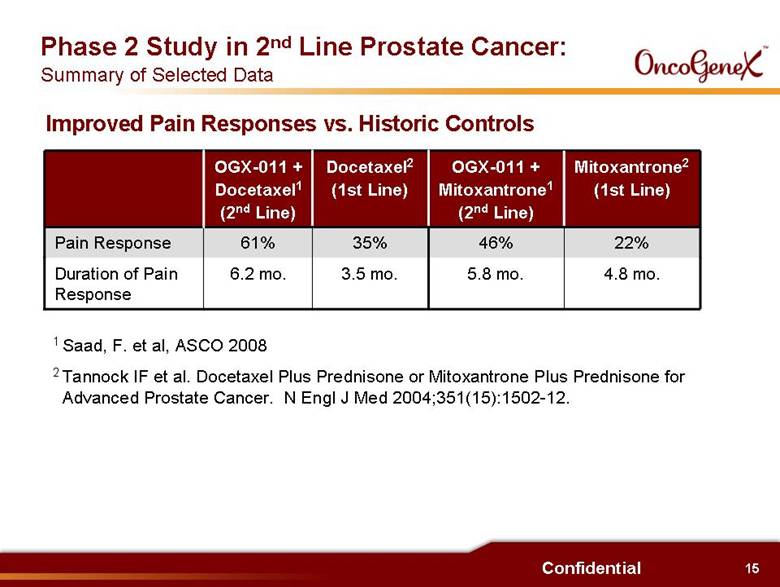
15 Confidential 1 Saad, F. et al, ASCO 2008 2 Tannock IF et al. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer. N Engl J Med 2004;351(15):1502-12. 3.5 mo. 35% Docetaxel2 (1st Line) 6.2 mo. 61% OGX-011 + Docetaxel1 (2nd Line) 22% 46% Pain Response Mitoxantrone2 (1st Line) OGX-011 + Mitoxantrone1 (2nd Line) 5.8 mo. 4.8 mo. Duration of Pain Response Improved Pain Responses vs. Historic Controls Phase 2 Study in 2nd Line Prostate Cancer: Summary of Selected Data |
|
|
16 Confidential Phase 2 Study in 2nd Line Prostate Cancer: Serum Clusterin Levels During OGX-011 Treatment Predictive of Survival The curves differ significantly (log-rank p = 0.0001) Survival Probability Survival Time (Months) 0 2 4 6 8 10 12 14 16 18 0 .2 .4 .6 .8 1 Low Average Clusterin During OGX-011 (n=35) Median Survival Time = 14.7 High Average Clusterin During OGX-011 Tx (n=7) Median Survival Time = 5.5 Low Average Clusterin During OGX-011 Tx (n=35) Median Survival Time = 14.7 Tx (n=7) |
|
|
17 Confidential 0 60 80 120 100 140 20 40 PSA Value Patient 025-011 Start of 1st line therapy End 1st line therapy 8 cycles PSA Progression OGX-011 2nd line therapy baseline OGX-011 treatment cycle 7 Time (2 weeks per mark) Phase 2 Study in 2nd Line Prostate Cancer: Evidence of Restoring Docetaxel Sensitivity with OGX-011 |
|
|
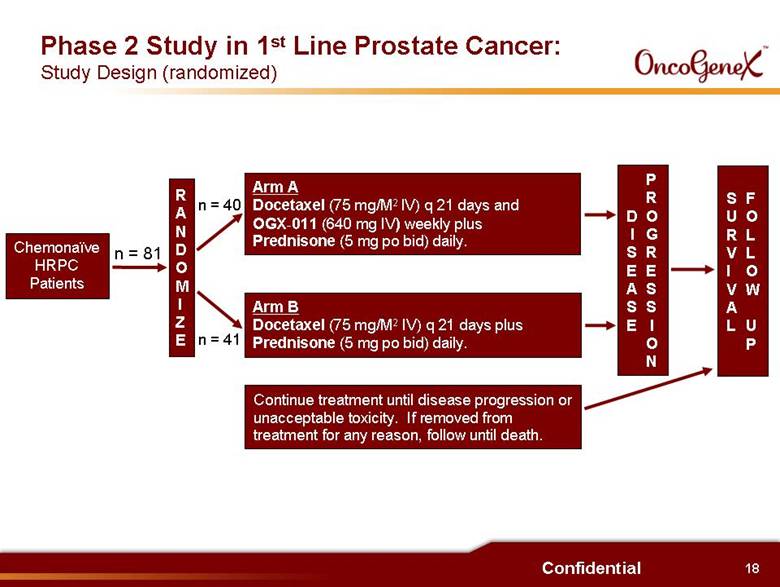
18 Confidential Chemonaïve HRPC Patients Phase 2 Study in 1st Line Prostate Cancer: Study Design (randomized) RANDOMIZE Arm A Docetaxel (75 mg/M2 IV) q 21 days and OGX-011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily. Arm B Docetaxel (75 mg/M2 IV) q 21 days plus Prednisone (5 mg po bid) daily. Continue treatment until disease progression or unacceptable toxicity. If removed from treatment for any reason, follow until death. DISESASE PROGRESSION SURVIVAL FOLLOW UP n = 81 n = 40 n = 41 |
|
|
19 Confidential Phase 2 Study in 1st Line Prostate Cancer: Favors OGX-011 + Docetaxel * 79% of patients are still alive 50% 77% Stable Measurable Disease 26.8% 2.5% Early Discontinuation of Chemotherapy 17% 4% Progressive Measurable Disease Docetaxel OGX-011 + docetaxel 2.6% 9 cycles 12% at 17.4 mo. 1st Line HR Prostate Cancer PSA Progression Number of Treatment Cycles Patient Deaths* 12.5% 7 cycles 29% at 17.7 mo. |
|
|
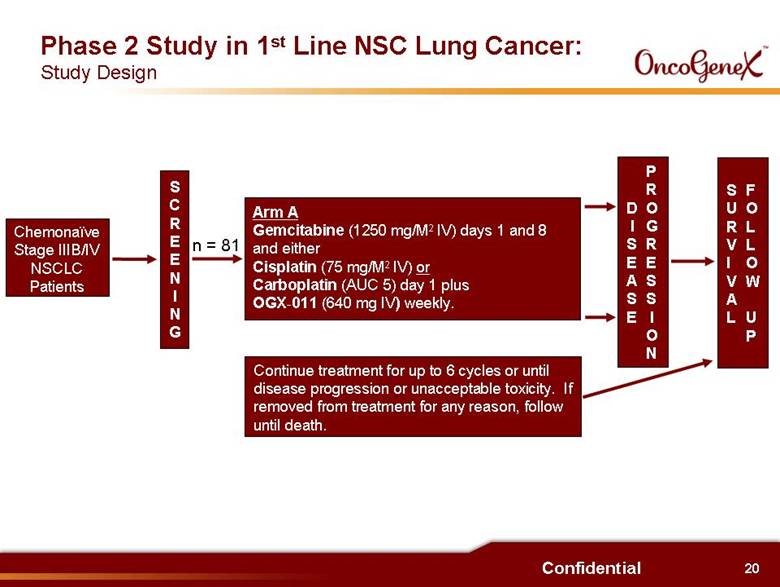
20 Confidential Chemonaïve Stage IIIB/IV NSCLC Patients Phase 2 Study in 1st Line NSC Lung Cancer: Study Design SCREENING Arm A Gemcitabine (1250 mg/M2 IV) days 1 and 8 and either Cisplatin (75 mg/M2 IV) or Carboplatin (AUC 5) day 1 plus OGX-011 (640 mg IV) weekly. Continue treatment for up to 6 cycles or until disease progression or unacceptable toxicity. If removed from treatment for any reason, follow until death. DISEASE PROGRESSION SURVIVAL FOLLOW UP n = 81 |
|
|
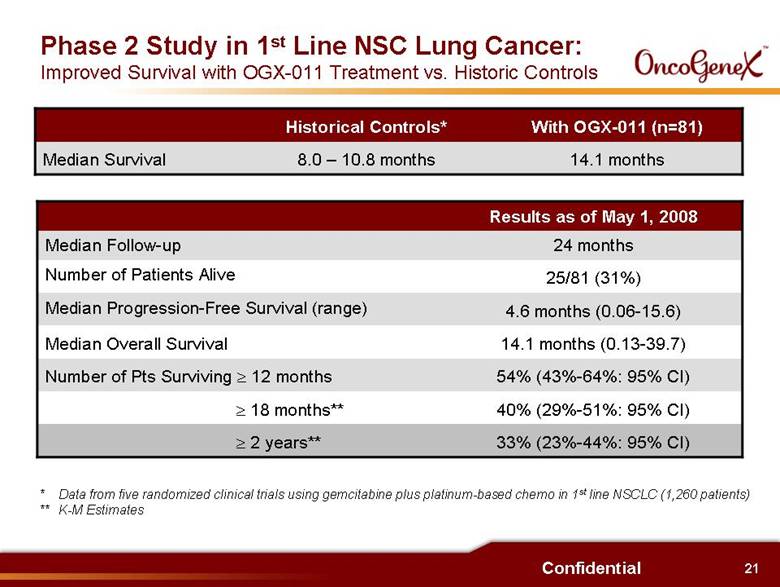
21 Confidential Phase 2 Study in 1st Line NSC Lung Cancer: Improved Survival with OGX-011 Treatment vs. Historic Controls 54% (43%-64%: 95% CI) Number of Pts Surviving > 12 months 14.1 months (0.13-39.7) Median Overall Survival 40% (29%-51%: 95% CI) > 18 months** 33% (23%-44%: 95% CI) > 2 years** 24 months Median Follow-up 4.6 months (0.06-15.6) Median Progression-Free Survival (range) 25/81 (31%) Number of Patients Alive Results as of May 1, 2008 With OGX-011 (n=81) Historical Controls* 14.1 months 8.0 10.8 months Median Survival * Data from five randomized clinical trials using gemcitabine plus platinum-based chemo in 1st line NSCLC (1,260 patients) ** K-M Estimates |
|
|
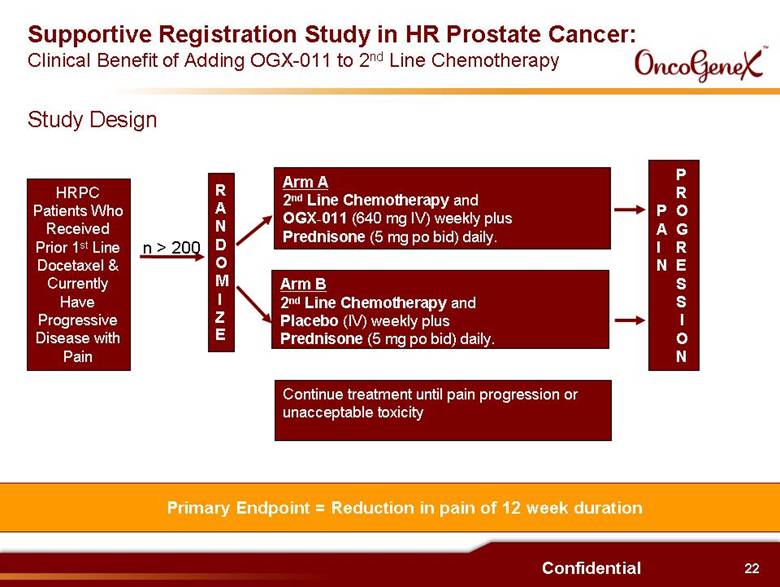
22 Confidential Supportive Registration Study in HR Prostate Cancer: Clinical Benefit of Adding OGX-011 to 2nd Line Chemotherapy RANDOMIZE Arm A 2nd Line Chemotherapy and OGX-011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily. Arm B 2nd Line Chemotherapy and Placebo (IV) weekly plus Prednisone (5 mg po bid) daily. Continue treatment until pain progression or unacceptable toxicity PAIN PROGRESSION n > 200 Primary Endpoint = Reduction in pain of 12 week duration HRPC Patients Who Received Prior 1st Line Docetaxel & Currently Have Progressive Disease with Pain Study Design |
|
|
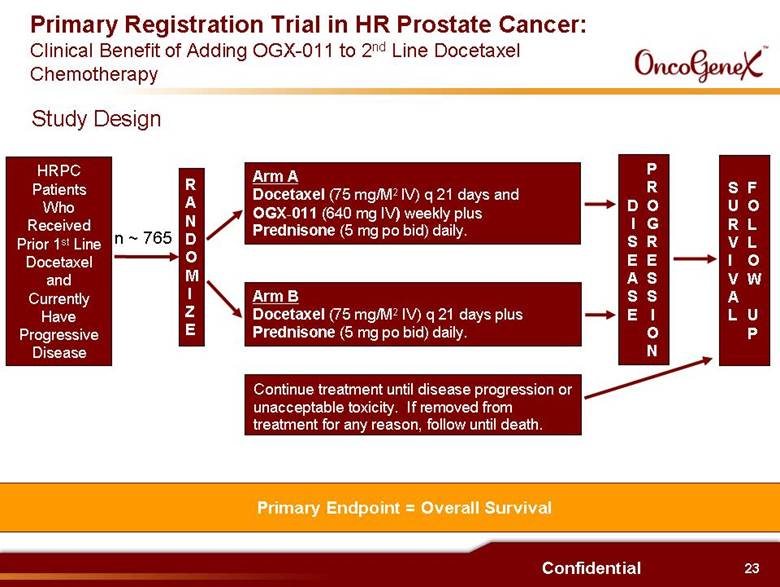
23 Confidential Primary Registration Trial in HR Prostate Cancer: Clinical Benefit of Adding OGX-011 to 2nd Line Docetaxel Chemotherapy RANDOMIZE Arm A Docetaxel (75 mg/M2 IV) q 21 days and OGX-011 (640 mg IV) weekly plus Prednisone (5 mg po bid) daily. Arm B Docetaxel (75 mg/M2 IV) q 21 days plus Prednisone (5 mg po bid) daily. Continue treatment until disease progression or unacceptable toxicity. If removed from treatment for any reason, follow until death. DISEASE PROGRESSION SURVIVAL FOLLOW UP n ~ 765 Primary Endpoint = Overall Survival HRPC Patients Who Received Prior 1st Line Docetaxel and Currently Have Progressive Disease Study Design |
|
|
24 Confidential OGX-011: Product Development Strategy . We have recently completed the Special Protocol Assessment with FDA for the primary registration trial which confirms prostate cancer patient population, study design and statistical plan . Obtain FDA input regarding reduction of pain as a primary endpoint . Initiate supportive registration trial in 1H 2009 Subject to availability of additional capital . The primary registration trials in each of HRPC and in NSCLC will be initiated when additional capital is available through partnering or financing Timing may follow completion of supportive registration trial |
|
|
25 Confidential OGX-427: Clinical Pipeline Opportunity Opportunity: Treatment resistant cancers including prostate, breast, lung, ovarian, bladder, pancreas, multiple myeloma, and others Target: Heat shock protein 27 (Hsp27) Mechanism of Action1: OGX-427 is designed to reduce the levels of Hsp27 which facilitates apoptosis by: Increasing Bax leading to increased Cytochrome C Increasing IK-B leading to decreased NF-KB Increasing protein aggregation leading to increased ER stress Decreasing IGF-1 and IL-6 signal transduction Increasing FasL mediated cell death Decreasing Androgen receptor activity Pre-clinical Data: Induces tumor cell death as monotherapy or in combination Delays tumor progression in multiple cancers Enhances anti-cancer activity with multiple chemotherapy agents Clinical Status: Phase 1 clinical trial ongoing 1 Assumed mechanism of action |
|
|
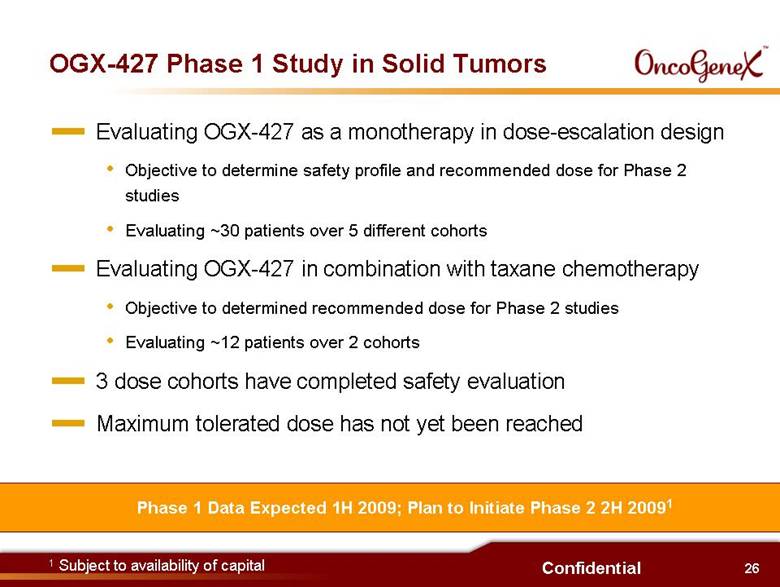
26 Confidential Evaluating OGX-427 as a monotherapy in dose-escalation design Objective to determine safety profile and recommended dose for Phase 2 studies Evaluating ~30 patients over 5 different cohorts Evaluating OGX-427 in combination with taxane chemotherapy Objective to determined recommended dose for Phase 2 studies Evaluating ~12 patients over 2 cohorts 3 dose cohorts have completed safety evaluation Maximum tolerated dose has not yet been reached OGX-427 Phase 1 Study in Solid Tumors Phase 1 Data Expected 1H 2009; Plan to Initiate Phase 2 2H 20091 1 Subject to availability of capital |
|
|
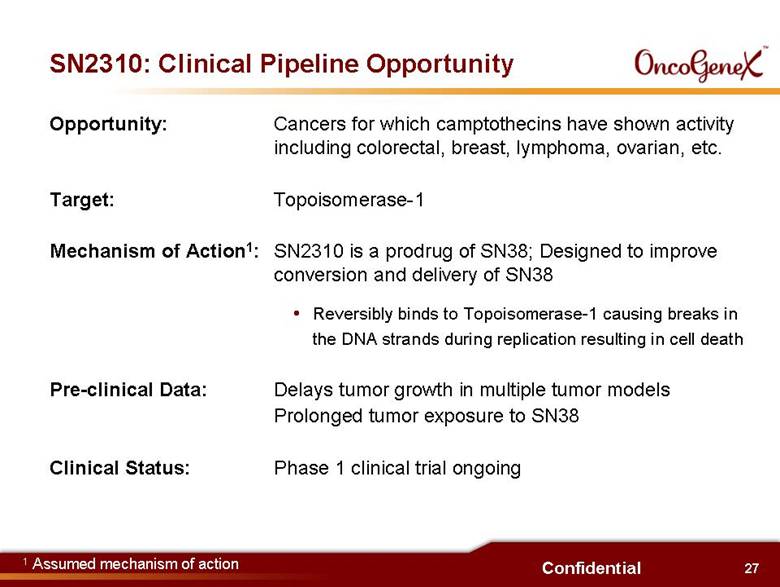
27 Confidential SN2310: Clinical Pipeline Opportunity Opportunity: Cancers for which camptothecins have shown activity including colorectal, breast, lymphoma, ovarian, etc. Target: Topoisomerase-1 Mechanism of Action1: SN2310 is a prodrug of SN38; Designed to improve conversion and delivery of SN38 Reversibly binds to Topoisomerase-1 causing breaks in the DNA strands during replication resulting in cell death Pre-clinical Data: Delays tumor growth in multiple tumor models Prolonged tumor exposure to SN38 Clinical Status: Phase 1 clinical trial ongoing 1 Assumed mechanism of action |
|
|
28 Confidential SN2310 Phase I Study in Solid Tumors Phase 1 Data Expected 1H 2009; Plan to Initiate Phase 2 in 2H 2009 1 Evaluating SN2310 as a monotherapy in dose-escalation design Objective to determine safety profile and recommended dose for Phase 2 studies 4 dose cohorts have completed safety evaluation Maximum tolerated dose has not yet been reached 1 Subject to availability of capital |
|
|
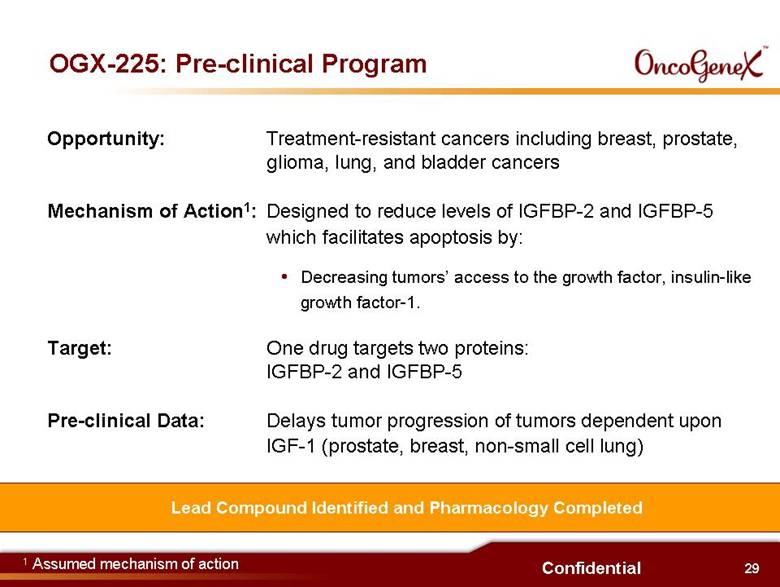
29 Confidential OGX-225: Pre-clinical Program Lead Compound Identified and Pharmacology Completed Opportunity: Treatment-resistant cancers including breast, prostate, glioma, lung, and bladder cancers Mechanism of Action1: Designed to reduce levels of IGFBP-2 and IGFBP-5 which facilitates apoptosis by: Decreasing tumors access to the growth factor, insulin-like growth factor-1 Target: One drug targets two proteins: IGFBP-2 and IGFBP-5 Pre-clinical Data: Delays tumor progression of tumors dependent upon IGF-1 (prostate, breast, non-small cell lung) 1 Assumed mechanism of action |
|
|
30 Confidential ~ $26M in cash and short term investments at the end of Q2 2008 Sufficient cash to achieve near-term development milestones: Financial Highlights of Combined Company Interim results for Phase 2 clinical trial with OGX-011 in 2nd Line HRPC 1H 2008 Interim results for Phase 2 clinical trial with OGX-011 in localized prostate cancer 1H 2008 Complete merger transaction and secure NASDAQ Listing 2H 2008 Complete SPA for primary registration trial of OGX-011 in 2nd Line HRPC 2H 2008 Obtain FDA input for additional registration trial of OGX-011 in 2nd Line HRPC 2H 2008 Survival data for Phase 2 clinical trial with OGX-011 in 1st Line HRPC 2H 2008 Determine MTD for SN2310 1H 2009 Determine MTD for OGX-427 as monotherapy 1H 2009 = Completed |
|
|
31 Confidential Financial Highlights of Combined Company (Continued) Additional financing will be required to extend the companys runway to enable achievement of long-term milestones Proceeds from any future financing anticipated to be used for: A supportive registration clinical trial for OGX-011 A randomized Phase 2 clinical trial for OGX-427 A randomized Phase 2 clinical trial for SN 2310 |
|
|
32 Confidential Proposed Timeline for Closing of Merger July 9, 2008: Definitive proxy statement mailed to Sonus shareholders August 11, 2008: OncoGenex shareholder meeting to approve merger August 19, 2008: Sonus shareholder meeting to approve merger August 20, 2008: Closing August 21, 2008: First day of trading as OncoGenex Pharmaceuticals, Inc (OGXI) The Board of Directors and Management of Sonus Recommend You Vote in Favor of All Resolutions Proposed for August 19 Shareholders Meeting |
|
|
33 Confidential OncoGenex Pharmaceuticals, Inc. A late-stage oncology company with lead product candidate focused on treatment-resistance Four unique product candidates approaching important development milestones: Lead product candidate in 5 Phase 2 clinical trials with plans to transition to supportive registration trial Two product candidates in Phase 1 with plans to transition to Phase 2 One pre-clinical product candidate with future plans to transition to Phase 1 Cash to support near term development milestones Experienced management with track record of product approvals Additional Information: www.oncogenex.ca or www.sonuspharma.com |
|
|
34 Confidential Proxy Solicitation In connection with the proposed merger, Sonus filed with the SEC a Proxy Statement and related materials on July 3, 2008 containing information about Sonus, OncoGenex and the proposed merger. Sonus mailed the Proxy Statement to its stockholders on or about July 9, 2008. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PROXY STATEMENT AND THE OTHER RELEVANT MATERIALS, CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY CONTAIN IMPORTANT INFORMATION ABOUT SONUS, ONCOGENEX AND THE PROPOSED MERGER. Sonus and OncoGenex, and certain of their directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sonus, including their respective security holdings, is set forth in Sonus Amendment No. 1 to Form 10-K for the fiscal year ended December 31, 2007, filed with the SEC on April 29, 2008, and the Proxy Statement filed with the SEC on July 3, 2008. As of June 30, 2008, OncoGenex directors and executive officers beneficially owned approximately 1,755,000 shares, or 14.5%, of OncoGenex capital stock. Investors may obtain additional information regarding the interests of OncoGenex, Sonus and their respective executive officers and directors in the merger by reading the Proxy Statement for such proposed transaction. The Proxy Statement and other relevant materials, and any other documents filed by Sonus with the SEC, may be obtained free of charge at the SEC's web site at www.sec.gov. In addition, investors and security holders may obtain free copies of the documents filed with the SEC by Sonus by directing a request to: Sonus Pharmaceuticals, Inc., 1522 217th Place SE, Suite 100, Bothell, WA 98021, Phone (425) 686-1500, Fax (425) 686-1600, Attention: Investor Relations. |
|
|
OncoGenex Pharmaceuticals, Inc. Merger of Sonus Pharmaceuticals Inc. and OncoGenex Technologies Inc. Committed to the development of new cancer therapies that address unmet needs in the treatment of cancer July 17, 2008 Bringing hope to life. |