DEFA14A: Additional definitive proxy soliciting materials and Rule 14(a)(12) material
Published on November 5, 2004
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a)
of the Securities Exchange Act of 1934
(Amendment No.__)
Filed by the Registrant o
Filed by a Party other than the Registrant o
Check the appropriate box:
o
|
Preliminary Proxy Statement | |
o
|
Confidential, for use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | |
o
|
Definitive Proxy Statement | |
o
|
Definitive Additional Materials | |
þ
|
Soliciting Material Under Rule 14a-12 |
SONUS PHARMACEUTICALS, INC.
Payment of Filing Fee (Check the appropriate box):
þ
|
No fee required. | |
o
|
Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | |
| 1) Title of each class of securities to which transaction applies: | ||
| 2) Aggregate number of securities to which transaction applies: | ||
| 3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11: | ||
| 4) Proposed maximum aggregate value of transaction: | ||
| 5) Total fee paid: | ||
o
|
Fee paid previously with preliminary materials. | |
o
|
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | |
| 1) Amount Previously Paid: | ||
| 2) Form, Schedule or Registration Statement No.: | ||
| 3) Filing Party: | ||
| 4) Date Filed: | ||
The following is a presentation given by Sonus Pharmaceuticals, Inc. (SNUS)
to investors during November 2004, and relates to the proposed acquisition of Synt:em, S.A.

| Synt:em Acquisition November 2004 |

| Forward-Looking Statement This presentation includes forward-looking statements such as those, among others, relating to the development, safety and efficacy of drug delivery products and potential applications for these products. As discussed in Sonus Pharmaceuticals' filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K filed on March 12, 2004 and Quarterly Report on Form 10-Q filed August 16, 2004, actual results could differ materially from those projected in the forward-looking statements as a result of the following factors, among others: the Company's and Synt:em's products will require extensive clinical testing and approval by regulatory authorities; such approvals are lengthy and expensive and may never occur; risks that the FDA may not approve the Company's proposed 505(b)(2) strategy; risks that clinical studies with TOCOSOL Paclitaxel will not be successful; risks that the Company may not be able to effectively or completely integrate the business and operations of Synt:em; risks that the combined company may not be able raise capital to finance the increased costs of the business and operations of both companies; and risks of successful development of additional drug delivery products. Sonus undertakes no obligation to update the forward looking statements contained herein or to reflect events or circumstances occurring after the date hereof. |

| Additional Information About the Acquisition and Where to Find It Sonus will file a proxy statement and other documents concerning the proposed acquisition of Synt:em with the Securities and Exchange Commission. Sonus stockholders are urged to read the proxy statement when it becomes available and other relevant documents filed with the SEC because they will contain important information. A copy of the proxy statement will be mailed to the stockholders of Sonus. Sonus stockholders may obtain a free copy of the proxy statement and other relevant documents filed by Sonus with the SEC when they become available at the SEC's website at www.sec.gov. The proxy statement and these other documents may also be obtained for free from Sonus by directing a request to: Investor Relations, 22026 20th Avenue S.E., Bothell, Washington, 98021, phone (425) 487-9500. Sonus and its directors, executive officers and certain of its employees may be deemed to be participants in the solicitation of proxies from the stockholders of Sonus with respect to the proposed transaction. Information regarding the names, affiliations and interests of the participants in the solicitation will be included in the proxy statement. |

| Agenda Strategic Rationale Synt:em Overview Deal Terms Financials Next Steps |

| Value Proposition Established strong foundation with TOCOSOL(r) Paclitaxel Sonus positioned to expand Explored significant number of opportunities to leverage Sonus capabilities and assets Acquisition results in stronger company positioned for increased shareholder value |

| TOCOSOL(r) Paclitaxel - Moving Ahead Discussions with FDA initiated on Phase 3 Proposing single pivotal trial Expect resolution by year end Partnership discussions gaining momentum Multinational pharmaceutical, specialty drug, and branded generic drug companies Term sheet stage with at least one prospect Planning to begin Phase 3 - Q2 '05 |

| Acquisition Benefits Pipeline Three near-term preclinical product candidates for pain management Expands oncology portfolio Platform Synergistic with TOCOSOL(r) drug delivery platform Drug discovery engine People Strong scientific expertise and collaborations Presence in Europe |

| Agenda Strategic Rationale Synt:em Overview Deal Terms Financials Next Steps |

| Synt:em Organization Founded in 1995 Based in Nimes, France Privately held 38 Employees Half hold doctoral degrees |

| Synt:em Pipeline Syn1002 Peptide analgesic for inflammatory and neuropathic pain Preclinical Very wide therapeutic window Total market est. at >$16 billion for '05 in U.S. (competes against COX-2 inhibitors) IND possibly in 2005 |

| Syn1002 Potency Inflammatory Pain Carrageenin Carrageenin + Syn1002 25 ug Carrageenin + morphine ( 4mg/kg) Carageenan PWD / heat model 3 h 6 h 0 2 4 6 8 Duration (s) Control Syn1002 is 160 times more potent than morphine |

| Syn1002 Potency Neuropathic Pain CCI Model N° of Paw withdrawals 0 1 2 3 4 5 6 7 8 9 10 Control Syn1002 (5 mg) Meloxicam (5mg/kg) Morphine (5 mg/kg) *** *** Syn1002 is 200 times more potent than morphine or meloxicam |

| Synt:em Pipeline Syn1003 Novel non-peptide opioid for acute/chronic pain Preclinical Good analgesic effects with potential for fewer side effects Market est. at >$12 billion in U.S. IND possibly in 2005 |

| Morphine SYN1003 candidate 0 2 4 6 8 10 12 0 60 120 180 240 300 360 420 Time (min) Latency Time (s) Syn1003 Analgesia Mice, Tail-Flick assay (IV bous, 5mg/kg.eq) SYN1003 candidate has a longer duration of action compare to morphine |

| Syn1003 Analgesia M6G Syn1003 candidate 0 1 2 3 4 5 6 7 8 9 10 0 30 60 90 120 150 180 210 240 270 300 330 360 390 420 Time ( min ) Latency Time ( s ) Mice, Tail-Flick assay (IV bous, 3mg/kg.eq)) Induction of constant analgesia requires less SYN1003 candidate than M6G |

| Synt:em Pipeline Syn1001 Novel opioid analgesic Preclinical Faster onset, longer duration of action, greater potency and enhanced CNS uptake Multi-billion dollar market opportunity for post-operative pain. IND possible in 2006 |

| Hotplate test: IV administration (1mg/kg eq) ANALGESIA* (% of mice) Time (min) Faster and prolonged analgesia compared to morphine and free M6G at the same dose Syn1001 Analgesic Effects * % of mice with a minimum of doubled latency time over baseline 10 Syn1001 Morphine 0 20 30 40 50 60 70 80 90 100 0 20 40 60 80 100 120 140 160 180 200 M6G |

| Syn1001 Lack of Respiratory Depression Morphine Free M6G Syn1001 40 50 60 70 80 90 100 0 30 60 90 120 150 180 Time (min) sO2 (% control) ED50 Morphine Free M6G Syn1001 40 50 60 70 80 90 100 0 30 60 90 120 150 180 Time (min) 5x ED50 SC Administration in rats Morphine Free M6G Syn1001 10x ED50 40 50 60 70 80 90 100 0 30 60 90 120 150 180 Time (min) |

| Synt:em Pipeline Oncology Syn2001 Brain cancer (glioblastoma multiforme) Syn2002 Lung cancer TAL-1 Solid tumors |

| Expanded Pipeline Discovery Pre-clinical Phase 1 Phase 2 Phase 3 Oncology TOCOSOL(r) Paclitaxel Camptothecin Platinum Syn 2001(Brain Cancer) Syn 2002 (Lung Cancer) Pain Management Syn 1002 Syn 1003 Syn 1001 |

| Synt:em Technology Platform Pep:trans(tm) Peptide chemistry for safe and efficient transport across complex cell membranes Can be linked to clinically validated or novel drugs |

| Pep:trans(tm) Re-engineering A way to change the pharmacological behavior of existing or new drugs Results in fully patentable new compounds Enables the improvement or expanded use of existing and new drugs Re-engineering of existing well-characterized drugs with Pep:trans creates new drugs faster Peptide Drug Linker |

| Synt:em Technology Platform Pep:trans(tm) Peptide chemistry for safe and efficient transport across complex cell membranes Can be linked to clinically validated or novel drugs Acti:map(tm) Validated computational process to discover and optimize selection of new peptides or drug candidates |

| Acti:map Pep:trans Combined Technology Platform Strategy Cell Vaccine MDR cancer Lung Cancer Syn2002 Asthma Inflammation Intestine Cancer Inflammation CNS Pain Syn1001 Infl. pain Syn1002 Cancer Syn 2001 Multiple Scl. Amyotrophic L. Scl. TM TM TOCOSOL(r) |
| Synt:em People Internal Team: Talented management team with excellent scientific credentials 6 key personnel; over half of research team holds doctoral degrees Complimentary competencies (very little overlap with Sonus capabilities) External team: Extensive network of scientific advisors and consultants Investors: Top tier, pan-European institutional shareholders |

| Synt:em Management Team Michel Kaczorek, Chairman and CEO. PhD, Biology Inst. Pasteur, Harvard MS, R&D Pasteur Vaccins, Proteine Performance. VP France Biotech Jamal Temsamani, Preclinical Development. PhD., Mol. Bio Worcester Foundation and Hybridon Roger Lahana, Drug Discovery. PhD, Chemistry CNRS, Molecular Chemistry, P. Fabre, Oxford Molecular (UK) Luc-Andre Granier, Medical Development. PhD, MD,Neurology Forenap, Eli Lilly Caroline Roussel, Operations. Ing. Biotechnology, EIEA Montpellier, Proteine Performance Gordon Waldron, Finance & Administration. Duke Univ., Spie Batignolles, Texas Instruments |

| Agenda Strategic Rationale Synt:em Overview Deal Terms Financials Next Steps |

| Summary of Transaction Stock-for-stock acquisition Total shares = $30M divided by closing price 1 Three stock payments - One at closing and two earn-out payments Total shares subject to an upper and lower collar Minimum shares issued if earn-out achieved - 7.6M Maximum shares issued if earn-out achieved - 8.9M 1 Assuming milestones are met Lockup for 9-18 mos. from issue date for all shares |

| Example of Shares Issued - Maximum Collar Shares issued - 8.9M share Payment New Shares Issued Cumulative Shares Issued Fully Diluted Shares Outstanding Synt:em Cumulative Ownership Sonus Ownership Initial Payment: $10.4M Based on $2.65 / Share 3,900,000 3,900,000 25,600,000 15.2% 84.8% Earn-out 1 50% of shares remaining after the Initial Payment 2,500,000 6,400,000 28,100,000 22.8% 77.2% Earn-out 2 50% of shares remaining after the Initial Payment 2,500,000 8,900,000 30,600,000 29.0% 71.0% |
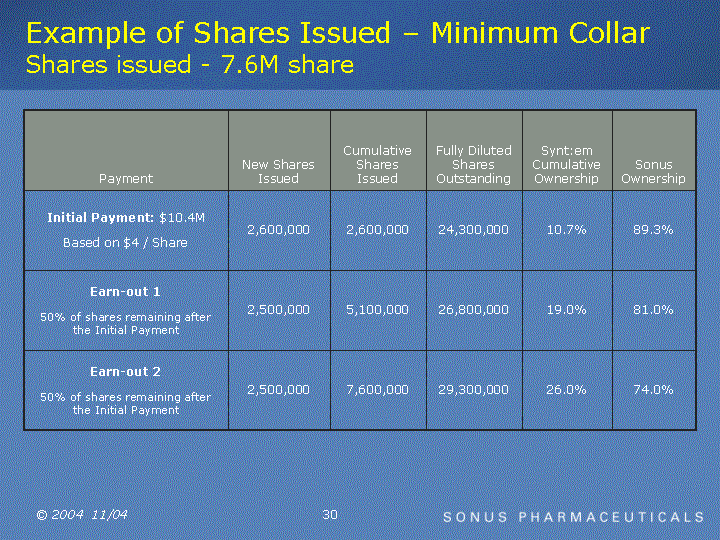
| Example of Shares Issued - Minimum Collar Shares issued - 7.6M share Payment New Shares Issued Cumulative Shares Issued Fully Diluted Shares Outstanding Synt:em Cumulative Ownership Sonus Ownership Initial Payment: $10.4M Based on $4 / Share 2,600,000 2,600,000 24,300,000 10.7% 89.3% Earn-out 1 50% of shares remaining after the Initial Payment 2,500,000 5,100,000 26,800,000 19.0% 81.0% Earn-out 2 50% of shares remaining after the Initial Payment 2,500,000 7,600,000 29,300,000 26.0% 74.0% |

| Attractive Comparison to Similar Transactions ($ in millions) At Closing Including Earnout Announced / Equity Enterprise Equity Enterprise Completed Acquirer / Target Value Value Value Value Stage / Indication 8/14/2003 8/21/2003 Genta / Salus $13.0 $12.7 $30.0 $29.7 Preclinical / Cancer 4/15/2003 8/21/2003 GenVec / Diacrin 42.1 4.5 42.1 4.5 Phase I / Cardio 2/10/2003 4/7/2003 Protein Design Labs / Eos 36.3 32.2 36.3 32.2 Preclinical / Cancer 11/12/2002 2/29/03 Incyte / Maxia 24.1 25.1 38.1 39.1 Preclinical / Diabetes 6/28/2001 7/6/2001 Sangamo / Gendaq 29.6 30.8 29.6 30.8 Preclinical / Discovery Mean $29.0 $21.1 $35.2 $27.3 Median 29.6 25.1 36.3 30.8 High 42.1 32.2 42.1 39.1 Low 13.0 4.5 29.6 4.5 Sonus / Synt:em $10.4 $8.0 $30.0 $27.6 Notes: Source: SDC Platinum, Company filings. (1) Additional $17.0M available upon achieving certain milestones, which have not been disclosed. (2) No earn out payment. (3) Additional $14.0M available upon achieving certain milestones, which have not been disclosed. (4) Values do not include contingent earn out payments. |

| Public Company Analysis |

| Sonus Pipeline - 2006 Discovery Pre-clinical Phase 1 Phase 2 Phase 3 Oncology TOCOSOL(r) Paclitaxel Camptothecin Platinum Syn 2001(Brain Cancer) Syn 2002 (Lung Cancer) Pain Management Syn 1002 Syn 1003 Syn 1001 |

| Agenda Strategic Rationale Synt:em Overview Deal Terms Financials Next Steps |

| Key Messages Near term Does not accelerate the need for cash/equity 3 new products in pipeline Intermediate term Requires additional funding, but Additional products in Phase 2 and Phase 3 Improved discovery engine Product offering beyond reformulation |

| Target Milestones Next 3 to 6 months Partnership on TOCOSOL(r) Paclitaxel Agreement with FDA on TOCOSOL(r) Paclitaxel Phase 3 program Initiation of Phase 3 testing for TOCOSOL(r) Paclitaxel Three additional products in preclinical Expanded pipeline |

| Agenda Strategic Rationale Synt:em Overview Deal Terms Financials Next Steps |

| Next Steps to Closing Proxy filing in mid November Proxy mailing in late December 1 Sonus shareholders meeting mid January 1 Close mid January 1 1 SEC review of proxy could delay six weeks. |

| A Stronger Sonus Building Greater Shareholder Value Pipeline Three near-term preclinical product candidates for pain management Expands oncology portfolio Platform Synergistic with TOCOSOL(tm) drug delivery platform Drug discovery engine People Strong scientific expertise and collaborations Presence in Europe |

| www.sonuspharma.com Nasdaq: SNUS |